Abstract
Purpose
The γ-aminobutyric acid (GABA) is the main inhibitory neurotransmitter and essential for normal brain function. The GABAergic system has been shown to have immunomodulatory effects and respond adaptively to excitatory toxicity. The association of the GABAergic system and inflammation in patients with multiple sclerosis (MS) remains unknown. In this pilot study, the in vivo relationship between GABAA binding and the innate immune response is explored using positron emission tomography (PET) with [11C] flumazenil (FMZ) and [11C]-PK11195 PET (PK-PET), a measure of activated microglia/macrophages.
Procedures
Sixteen MS patients had dynamic FMZ-PET and PK-PET imaging. Ten age-matched healthy controls (HC) had a single FMZ-PET. GABAA receptor binding was calculated using Logan reference model with the pons as reference. Distribution of volume ratio (VTr) for PK-PET was calculated using image-derived input function. A hierarchical linear model was fitted to assess the linear association between PK-PET and FMZ-PET among six cortical regions of interest.
Results
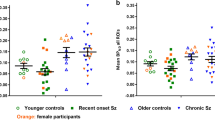
GABAA receptor binding was higher throughout the cortex in MS patients (5.72 ± 0.91) as compared with HC (4.70 ± 0.41) (p = 0.002). A significant correlation was found between FMZ binding and PK-PET within the cortex (r = 0.61, p < 0.001) and among the occipital (r = 0.61, p = 0.012), parietal (r = 0.49, p = 0.041), and cingulate (r = 0.32, p = 0.006) regions.
Conclusions
A higher GABAA receptor density in MS subjects compared with HC was observed and correlated with innate immune activity. Our observations demonstrate that immune-driven GABAergic abnormalities may be present in MS.




Similar content being viewed by others
References
Araujo SES, Mendonca HR, Wheeler NA et al (2017) Inflammatory demyelination alters subcortical visual circuits. J Neuroinflammation 14:162
Cawley N, Solanky BS, Muhlert N, Tur C, Edden RAE, Wheeler-Kingshott CAM, Miller DH, Thompson AJ, Ciccarelli O (2015) Reduced gamma-aminobutyric acid concentration is associated with physical disability in progressive multiple sclerosis. Brain 138:2584–2595
Nantes JC, Proulx S, Zhong J, Holmes SA, Narayanan S, Brown RA, Hoge RD, Koski L (2017) GABA and glutamate levels correlate with MTR and clinical disability: insights from multiple sclerosis. NeuroImage 157:705–715
Freeman L, Garcia-Lorenzo D, Bottin L, Leroy C, Louapre C, Bodini B, Papeix C, Assouad R, Granger B, Tourbah A, Dollé F, Lubetzki C, Bottlaender M, Stankoff B (2015) The neuronal component of gray matter damage in multiple sclerosis: a [(11) C]flumazenil positron emission tomography study. Ann Neurol 78:554–567
Wang DS, Zurek AA, Lecker I, Yu J, Abramian AM, Avramescu S, Davies PA, Moss SJ, Lu WY, Orser BA (2012) Memory deficits induced by inflammation are regulated by alpha5-subunit-containing GABAA receptors. Cell Rep 2:488–496
Serantes R, Arnalich F, Figueroa M, Salinas M, Andrés-Mateos E, Codoceo R, Renart J, Matute C, Cavada C, Cuadrado A, Montiel C (2006) Interleukin-1beta enhances GABAA receptor cell-surface expression by a phosphatidylinositol 3-kinase/Akt pathway: relevance to sepsis-associated encephalopathy. J Biol Chem 281:14632–14643
Bibolini MJ, Chanaday NL, Baez NS, Degano AL, Monferran CG, Roth GA (2011) Inhibitory role of diazepam on autoimmune inflammation in rats with experimental autoimmune encephalomyelitis. Neuroscience 199:421–428
Lee M, Schwab C, McGeer PL (2011) Astrocytes are GABAergic cells that modulate microglial activity. Glia 59:152–165
Bhat R, Axtell R, Mitra A, Miranda M, Lock C, Tsien RW, Steinman L (2010) Inhibitory role for GABA in autoimmune inflammation. Proc Natl Acad Sci U S A 107:2580–2585
Odano I, Halldin C, Karlsson P, Varrone A, Airaksinen AJ, Krasikova RN, Farde L (2009) [18F]flumazenil binding to central benzodiazepine receptor studies by PET-quantitative analysis and comparisons with [11C]flumazenil. NeuroImage 45:891–902
Maziere M, Hantraye P, Prenant C, Sastre J, Comar D (1984) Synthesis of ethyl 8-fluoro-5,6-dihydro-5-[11C]methyl-6-oxo-4H-imidazo [1,5-a] [1,4]benzodiazepine-3-carboxylate (RO 15.1788-11C): a specific radioligand for the in vivo study of central benzodiazepine receptors by positron emission tomography. Int J Appl Radiat Isot 35:973–976
Heiss WD, Sobesky J, Smekal U et al (2004) Probability of cortical infarction predicted by flumazenil binding and diffusion-weighted imaging signal intensity: a comparative positron emission tomography/magnetic resonance imaging study in early ischemic stroke. Stroke 35:1892–1898
Lamusuo S, Pitkanen A, Jutila L, Ylinen A, Partanen K, Kalviainen R, Ruottinen HM, Oikonen V, Nagren K, Lehikoinen P, Vapalahti M, Vainio P, Rinne JO (2000) [11 C]Flumazenil binding in the medial temporal lobe in patients with temporal lobe epilepsy: correlation with hippocampal MR volumetry, T2 relaxometry, and neuropathology. Neurology 54:2252–2260
Geeraerts T, Coles JP, Aigbirhio FI, Pickard JD, Menon DK, Fryer TD, Hong YT (2011) Validation of reference tissue modelling for [11C]flumazenil positron emission tomography following head injury. Ann Nucl Med 25:396–405
Gandhi R, Laroni A, Weiner HL (2010) Role of the innate immune system in the pathogenesis of multiple sclerosis. J Neuroimmunol 221:7–14
Banati RB, Newcombe J, Gunn RN, Cagnin A, Turkheimer F, Heppner F, Price G, Wegner F, Giovannoni G, Miller DH, Perkin GD, Smith T, Hewson AK, Bydder G, Kreutzberg GW, Jones T, Cuzner ML, Myers R (2000) The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity. Brain 123(Pt 11):2321–2337
Politis M, Giannetti P, Su P, Turkheimer F, Keihaninejad S, Wu K, Waldman A, Malik O, Matthews PM, Reynolds R, Nicholas R, Piccini P (2012) Increased PK11195 PET binding in the cortex of patients with MS correlates with disability. Neurology 79:523–530
Rissanen E, Tuisku J, Rokka J, Paavilainen T, Parkkola R, Rinne JO, Airas L (2014) In vivo detection of diffuse inflammation in secondary progressive multiple sclerosis using PET imaging and the radioligand (1)(1)C-PK11195. Eur J Nucl Med 55:939–944
Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage 9:179–194
Kang Y, Schlyer D, Kaunzner UW, Kuceyeski A, Kothari PJ, Gauthier SA (2018) Comparison of two different methods of image analysis for the assessment of microglial activation in patients with multiple sclerosis using (R)-[N-methyl-carbon-11] PK11195. PLoS One 13:e0201289
Mikolajczyk K, Szabatin M, Rudnicki P, Grodzki M, Burger C (1998) A JAVA environment for medical image data analysis: initial application for brain PET quantitation. Med Inf 23:207–214
Hashimoto K, Inoue O, Suzuki K, Yamasaki T, Kojima M (1989) Synthesis and evaluation of 11C-PK 11195 for in vivo study of peripheral-type benzodiazepine receptors using positron emission tomography. Ann Nucl Med 3:63–71
Kang Y, Mozley PD, Verma A, Schlyer D, Henchcliffe C, Gauthier SA, Chiao PC, He B, Nikolopoulou A, Logan J, Sullivan JM, Pryor KO, Hesterman J, Kothari PJ, Vallabhajosula S (2018) Noninvasive PK11195-PET image analysis techniques can detect abnormal cerebral microglial activation in Parkinson’s disease. J Neuroimaging 28:496–505
Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL (1996) Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 16:834–840
Logan J (2000) Graphical analysis of PET data applied to reversible and irreversible tracers. Nucl Med Biol 27:661–670
Kaunzner UW, Kang Y, Zhang S, Morris E, Yao Y, Pandya S, Hurtado Rua SM, Park C, Gillen KM, Nguyen TD, Wang Y, Pitt D, Gauthier SA (2019) Quantitative susceptibility mapping identifies inflammation in a subset of chronic multiple sclerosis lesions. Brain 142:133–145
Team RC R: A language and environment for statistical computing. URL http://www.R-project.org/
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate-a practical and powerful approach to multiple testing. J Roy Stat Soc B Met 57:289–300
LoCastro E, Kuceyeski A, Raj A (2014) Brainography: an atlas-independent surface and network rendering tool for neural connectivity visualization. Neuriinformatics 12:355–359
Fritschy JM, Panzanelli P (2014) GABAA receptors and plasticity of inhibitory neurotransmission in the central nervous system. Eur J Neurosci 39:1845–1865
Li Y, Sun H, Chen Z, Xu H, Bu G, Zheng H (2016) Implications of GABAergic neurotransmission in Alzheimer’s disease. Front Aging Neurosci 8:31
Dutta R, McDonough J, Yin X, Peterson J, Chang A, Torres T, Gudz T, Macklin WB, Lewis DA, Fox RJ, Rudick R, Mirnics K, Trapp BD (2006) Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann Neurol 59:478–489
Accardi MV, Daniels BA, Brown PM, Fritschy JM, Tyagarajan SK, Bowie D (2014) Mitochondrial reactive oxygen species regulate the strength of inhibitory GABA-mediated synaptic transmission. Nat Commun 5:3168
Kiljan S, Prins M, Baselmans BM, Bol J, Schenk GJ, van Dam AM (2019) Enhanced GABAergic immunoreactivity in hippocampal neurons and astroglia of multiple sclerosis patients. J Neuropathol Exp Neurol 78:480–491
Sarchielli P, Greco L, Floridi A, Floridi A, Gallai V (2003) Excitatory amino acids and multiple sclerosis: evidence from cerebrospinal fluid. Arch Neurol 60:1082–1088
Newcombe J, Uddin A, Dove R, Patel B, Turski L, Nishizawa Y, Smith T (2008) Glutamate receptor expression in multiple sclerosis lesions. Brain Pathol 18:52–61
Azevedo CJ, Kornak J, Chu P, Sampat M, Okuda DT, Cree BA, Nelson SJ, Hauser SL, Pelletier D (2014) In vivo evidence of glutamate toxicity in multiple sclerosis. Ann Neurol 76:269–278
Vercellino M, Merola A, Piacentino C, Votta B, Capello E, Mancardi GL, Mutani R, Giordana MT, Cavalla P (2007) Altered glutamate reuptake in relapsing-remitting and secondary progressive multiple sclerosis cortex: correlation with microglia infiltration, demyelination, and neuronal and synaptic damage. J Neuropathol Exp Neurol 66:732–739
Andersson JD, Matuskey D, Finnema SJ (2018) Positron emission tomography imaging of the gamma-aminobutyric acid system. Neurosci Lett
Frankle WG, Cho RY, Narendran R, Mason NS, Vora S, Litschge M, Price JC, Lewis DA, Mathis CA (2009) Tiagabine increases [11C]flumazenil binding in cortical brain regions in healthy control subjects. Neuropsychopharmacology 34:624–633
Cosenza-Nashat M, Zhao ML, Suh HS, Morgan J, Natividad R, Morgello S, Lee SC (2009) Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol Appl Neurobiol 35:306–328
Zurawski J, Tauhid S, Chu R, Khalid F, Healy BC, Weiner HL, Bakshi R (2020) 7T MRI cerebral leptomeningeal enhancement is common in relapsing-remitting multiple sclerosis and is associated with cortical and thalamic lesions. Mult Scler 26:177–187
Mandolesi G, Gentile A, Musella A, Fresegna D, de Vito F, Bullitta S, Sepman H, Marfia GA, Centonze D (2015) Synaptopathy connects inflammation and neurodegeneration in multiple sclerosis. Nat Rev Neurol 11:711–724
Acknowledgments
The authors are grateful to the professional staff at the Citigroup Biomedical Imaging Center of Weill Cornell Medical College, including, in alphabetical order, John W. Babich PhD, Jonathan Dyke PhD, Simon Morim, Amelia Ng, and Nelsie Pastrano-Redula. The authors also want to acknowledge Sneha Pandya and David Schlyer PhD for advisement on data analysis. The authors further acknowledge Sudhin A. Shah PhD with regard to health control subject recruitment and data sharing. The authors acknowledge Leorah Freeman MD for her thoughtful review of the manuscript. This conduct of the research was supported by in part through an investigator-initiated study supported by Biogen and the Weill Cornell Clinical and Translational Science Center (CTSC).
Funding
This work was supported by in part through an investigator-initiated study supported by Biogen Cambridge MA. (grant # 55000025) and from the Weill Cornell Clinical and Translational Science Center (CTSC), New York, NY (grant UL1 TR000456-06).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Kang reports no disclosures. Dr. Kaunzner reports no disclosures. Dr. Perumal reports no disclosures. Dr. Nealon reports no disclosures. Dr. Perumal has received consultant fees from Genzyme. Dr. Kothari reports no disclosures. Dr. Vartanian has received consultant fees from Biogen, Norvartis, and Genzyme as well as Celgene. Dr. Kuceyeski reports no disclosures. Hurtado Rúa reports no disclosures. Dr. Gauthier has grant support from Genzyme, Genentech, and Mallinckrodt and has also received consulting fees from Biogen, Genetech, and Celgene.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material

Supplement Fig. 1
Illustrate time activity curves of Pons with MS patients and HC. There is no significant difference in any time points. (JPG 39 kb)
ESM 2
(DOCX 59 kb)
Rights and permissions
About this article
Cite this article
Kang, Y., Rúa, S.M.H., Kaunzner, U.W. et al. A Multi-Ligand Imaging Study Exploring GABAergic Receptor Expression and Inflammation in Multiple Sclerosis. Mol Imaging Biol 22, 1600–1608 (2020). https://doi.org/10.1007/s11307-020-01501-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-020-01501-z