Abstract
Descending command neurons instruct spinal networks to execute basic locomotor functions, such as gait and speed. The command functions for gait and speed are symmetric, implying that a separate unknown system directs asymmetric movements, including the ability to move left or right. In the present study, we report that Chx10-lineage reticulospinal neurons act to control the direction of locomotor movements in mammals. Chx10 neurons exhibit mainly ipsilateral projection, and their selective unilateral activation causes ipsilateral turning movements in freely moving mice. Unilateral inhibition of Chx10 neurons causes contralateral turning movements. Paired left–right motor recordings identified distinct mechanisms for directional movements mediated via limb and axial spinal circuits. Finally, we identify sensorimotor brain regions that project on to Chx10 reticulospinal neurons, and demonstrate that their unilateral activation can impart left–right directional commands. Together these data identify the descending motor system that commands left–right locomotor asymmetries in mammals.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
The code used to analyze data and produce figure content is available from the corresponding author upon request.
References
Grillner, S. The motor infrastructure: from ion channels to neuronal networks. Nat. Rev. Neurosci. 4, 573–586 (2003).
Kiehn, O. Decoding the organization of spinal circuits that control locomotion. Nat. Rev. Neurosci. 17, 224–238 (2016).
Goulding, M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat. Rev. Neurosci. 10, 507–518 (2009).
Brownstone, R. M. & Wilson, J. M. Strategies for delineating spinal locomotor rhythm-generating networks and the possible role of Hb9 interneurones in rhythmogenesis. Brain Res. Rev. 57, 64–76 (2008).
Jordan, L. M., Liu, J., Hedlund, P. B., Akay, T. & Pearson, K. G. Descending command systems for the initiation of locomotion in mammals. Brain Res. Rev. 57, 183–191 (2008).
Ryczko, D. & Dubuc, R. The multifunctional mesencephalic locomotor region. Curr. Pharm. Des. 19, 4448–4470 (2013).
Capelli, P., Pivetta, C., Soledad Esposito, M. & Arber, S. Locomotor speed control circuits in the caudal brainstem. Nature 551, 373–377 (2017).
Caggiano, V. et al. Midbrain circuits that set locomotor speed and gait selection. Nature 553, 455–460 (2018).
Josset, N. et al. Distinct contributions of mesencephalic locomotor region nuclei to locomotor control in the freely behaving mouse. Curr. Biol. 28, 884–901.e3 (2018).
Musienko, P. E. et al. Spinal and supraspinal control of the direction of stepping during locomotion. J. Neurosci. 32, 17442–17453 (2012).
Roseberry, T. K. et al. Cell-type-specific control of brainstem locomotor circuits by basal ganglia. Cell 164, 526–537 (2016).
Bouvier, J. et al. Descending command neurons in the brainstem that halt locomotion. Cell 163, 1191–1203 (2015).
Juvin, L. et al. A specific population of reticulospinal neurons controls the termination of locomotion. Cell Rep. 15, 2377–2386 (2016).
Grillner, S. et al. Intrinsic function of a neuronal network—a vertebrate central pattern generator. Brain Res. Brain Res. Rev. 26, 184–197 (1998).
Brocard, F. et al. The transformation of a unilateral locomotor command into a symmetrical bilateral activation in the brainstem. J. Neurosci. 30, 523–533 (2010).
Shik, M. L., Severin, F. V. & Orlovskiĭ, G. N. Control of walking and running by means of electric stimulation of the midbrain. Biofizika 11, 659–666 (1966).
Shefchyk, S. J., Jell, R. M. & Jordan, L. M. Reversible cooling of the brainstem reveals areas required for mesencephalic locomotor region evoked treadmill locomotion. Exp. Brain Res. 56, 257–262 (1984).
Garcia-Rill, E. & Skinner, R. D. The mesencephalic locomotor region. I. Activation of a medullary projection site. Brain Res. 411, 1–12 (1987).
Garcia-Rill, E. & Skinner, R. D. The mesencephalic locomotor region. II. Projections to reticulospinal neurons. Brain Res. 411, 13–20 (1987).
Bachmann, L. C. et al. Deep brain stimulation of the midbrain locomotor region improves paretic hindlimb function after spinal cord injury in rats. Sci. Transl. Med. 5, 208ra146 (2013).
Ferreira-Pinto, M. J., Ruder, L., Capelli, P. & Arber, S. Connecting circuits for supraspinal control of locomotion. Neuron 100, 361–374 (2018).
Steinmetz, N. A., Zatka-Haas, P., Carandini, M. & Harris, K. D. Distributed coding of choice, action and engagement across the mouse brain. Nature 576, 266–273 (2019).
Hagglund, M. et al. Optogenetic dissection reveals multiple rhythmogenic modules underlying locomotion. Proc. Natl Acad. Sci. USA 110, 11589–11594 (2013).
Kjaerulff, O. & Kiehn, O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J. Neurosci. 16, 5777–5794 (1996).
Bracci, E., Ballerini, L. & Nistri, A. Localization of rhythmogenic networks responsible for spontaneous bursts induced by strychnine and bicuculline in the rat isolated spinal cord. J. Neurosci. 16, 7063–7076 (1996).
Bonnot, A., Whelan, P. J., Mentis, G. Z. & O’Donovan, M. J. Locomotor-like activity generated by the neonatal mouse spinal cord. Brain Res. Brain Res. Rev. 40, 141–151 (2002).
Kremer, E. & Lev-Tov, A. Localization of the spinal network associated with generation of hindlimb locomotion in the neonatal rat and organization of its transverse coupling system. J. Neurophysiol. 77, 1155–1170 (1997).
Muir, G. D. & Whishaw, I. Q. Complete locomotor recovery following corticospinal tract lesions: measurement of ground reaction forces during overground locomotion in rats. Behav. Brain Res. 103, 45–53 (1999).
Jin, D. et al. Restoration of skilled locomotion by sprouting corticospinal axons induced by co-deletion of PTEN and SOCS3. Nat. Commun. 6, 8074 (2015).
Deliagina, T. G., Popova, L. B. & Grant, G. The role of tonic vestibular input for postural control in rats. Arch. Ital. Biol. 135, 239–261 (1997).
Armbruster, B. N., Li, X., Pausch, M. H., Herlitze, S. & Roth, B. L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl Acad. Sci. USA 104, 5163–5168 (2007).
Hägglund, M., Borgius, L., Dougherty, K. J. & Kiehn, O. Activation of groups of excitatory neurons in the mammalian spinal cord or hindbrain evokes locomotion. Nat. Neurosci. 13, 246–252 (2010).
Kjaerulff, O. & Kiehn, O. Crossed rhythmic synaptic input to motoneurons during selective activation of the contralateral spinal locomotor network. J. Neurosci. 17, 9433–9447 (1997).
Mathis, A. et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 21, 1281–1289 (2018).
Bellardita, C. & Kiehn, O. Phenotypic characterization of speed-associated gait changes in mice reveals modular organization of locomotor networks. Curr. Biol. 25, 1426–1436 (2015).
Wall, N. R., Wickersham, I. R., Cetin, A., De La Parra, M. & Callaway, E. M. Monosynaptic circuit tracing in vivo through Cre-dependent targeting and complementation of modified rabies virus. Proc. Natl Acad. Sci. USA 107, 21848–21853 (2010).
Liu, K. et al. Lhx6-positive GABA-releasing neurons of the zona incerta promote sleep. Nature 548, 582–587 (2017).
Zingg, B. et al. AAV-mediated anterograde transsynaptic tagging: mapping corticocollicular input-defined neural pathways for defense behaviors. Neuron 93, 33–47 (2017).
Shang, C. et al. A parvalbumin-positive excitatory visual pathway to trigger fear responses in mice. Science 348, 1472–1477 (2015).
Felsen, G. & Mainen, Z. F. Neural substrates of sensory-guided locomotor decisions in the rat superior colliculus. Neuron 60, 137–148 (2008).
Oliveira, A. F. & Yonehara, K. The mouse superior colliculus as a model system for investigating cell type-based mechanisms of visual motor transformation. Front. Neural Circuits 12, 59 (2018).
Borgius, L., Restrepo, C. E., Leao, R. N., Saleh, N. & Kiehn, O. A transgenic mouse line for molecular genetic analysis of excitatory glutamatergic neurons. Mol. Cell. Neurosci. 45, 245–257 (2010).
Fagerstedt, P., Orlovsky, G. N., Deliagina, T. G., Grillner, S. & Ullén, F. Lateral turns in the lamprey. ii. Activity of reticulospinal neurons during the generation of fictive turns. J. Neurophysiol. 86, 2257–2265 (2001).
Huang, K. H., Ahrens, M. B., Dunn, T. W. & Engert, F. Spinal projection neurons control turning behaviors in zebrafish. Curr. Biol. 23, 1566–1573 (2013).
Kozlov, A. K., Kardamakis, A. A., Hellgren Kotaleski, J. & Grillner, S. Gating of steering signals through phasic modulation of reticulospinal neurons during locomotion. Proc. Natl Acad. Sci. USA 111, 3591–3596 (2014).
Thiele, T. R., Donovan, J. C. & Baier, H. Descending control of swim posture by a midbrain nucleus in zebrafish. Neuron 83, 679–691 (2014).
Gruntman, E., Benjamini, Y. & Golani, I. Coordination of steering in a free-trotting quadruped. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 193, 331–345 (2007).
Kimura, Y. alx, a zebrafish homolog of Chx10, marks ipsilateral descending excitatory interneurons that participate in the regulation of spinal locomotor circuits. J. Neurosci. 26, 5684–5697 (2006).
Bagnall, M. W. et al. Glycinergic projection neurons of the cerebellum. J. Neurosci. 29, 10104–10110 (2009).
Muzzu, T., Mitolo, S., Gava, G. P. & Schultz, S. R. Encoding of locomotion kinematics in the mouse cerebellum. PLoS ONE 13, e0203900 (2018).
Azim, E., Jiang, J., Alstermark, B. & Jessell, T. M. Skilled reaching relies on a V2a propriospinal internal copy circuit. Nature 508, 357–363 (2014).
Oh, S. W. et al. A mesoscale connectome of the mouse brain. Nature 508, 207–214 (2014).
Murray, A. J. et al. Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat. Neurosci. 14, 297–299 (2011).
Wickersham, I. R. & Sullivan, H. A. Rabies viral vectors for monosynaptic tracing and targeted transgene expression in neurons. Cold Spring Harb. Protoc. 4, 375–385 (2015).
Franklin, K. B. J. & Paxinos, G. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates (Academic Press, 2019).
Bellardita, C. et al. Spatiotemporal correlation of spinal network dynamics underlying spasms in chronic spinalized mice. eLife 6, e23011 (2017).
Nath, T. et al. Using DeepLabCut for 3D markerless pose estimation across species and behaviors. Nat. Protoc. 14, 2152–2176 (2019).
Lein, E. S. et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 (2007).
Jennings, J. H. et al. Interacting neural ensembles in orbitofrontal cortex for social and feeding behaviour. Nature 565, 645–649 (2019).
Evans, D. A. et al. A synaptic threshold mechanism for computing escape decisions. Nature 558, 590–594 (2018).
Acknowledgements
We thank K. Sharma, L. Zagoraiou, S. Crone and T.M. Jessell for the Chx10Cre mouse line. We acknowledge the Core Facility for Integrated Microscopy, Faculty of Health and Medical Sciences, University of Copenhagen. We thank I. Vesth-Hansen and D. Meinertz for technical assistance, and members of O.K.’s laboratory for discussion and comments on previous versions of this manuscript. This work was supported by an EMBO Long-Term Fellowship (to J.M.C., grant no. ALTF 421-2018), the European Research Council under the European Union’s Horizon 2020 research and innovation program (to O.K., grant agreement no. REP-SCI-693038), the Novo Nordisk Laureate Program (to O.K., NNF15OC0014186), the Faculty of Health and Medical Sciences University of Copenhagen (to O.K. and A.M.) and the Swedich Resarch Council (to O.K.).
Author information
Authors and Affiliations
Contributions
J.M.C. and O.K. conceptualized the study. J.M.C., R.L., A.M. and O.K. provided methodology. J.M.C., R.L., A.M. and P.W. carried out the investigations. I.R.W. provided resources. J.M.C. and O.K. wrote the original draft. J.M.C., R.L. and O.K. wrote, reviewed and edited the manuscript. O.K. supervised the study. J.M.C. and O.K. acquired funds.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Neuroscience thanks Michael Fehlings and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
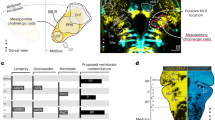
Extended Data Fig. 1 Chx10 Gi neurons form a prominent tract of ipsilaterally projecting axons.
a, Unilateral labeling of Chx10 neurons of the rostral gigantocellularis (Gi) using the Cre-dependent anterograde tracer AAV-FLEX-tdTom-2A-synGFP. b, Top, Sagittal section of tdTom+ projections ipsilateral to the injection site. tdTom+ axons formed a prominent tract that projected caudally to the spinal cord. Bottom, inset from Top. XII, hypoglossal motor nucleus; pyx, pyramidal decussation; IO, inferior olive; PMC, phrenic motor column; MMC, medial motor column. Images in (b) are representative of tracing experiments from n = 3 mice. c, Coalescence of Chx10 reticulospinal axons dorsal to the inferior olive (top), and subsequent positioning in the ventrolateral funiculus at the level of the pyramidal decussation (bottom). Images in (c) are representative of tracing experiments from n = 6 mice.
Extended Data Fig. 2 CNO administration does not affect turning preference in control AAV-mCherry injected mice.
a, Example of mCherry expression 3 weeks after a 500 nl unilateral injection of AAV-FLEX-mCherry in a Chx10Cre mouse (representative image from n = 6 mice). mCherry expression is confined to the side ipsilateral to injection. b, Turning preference in a 10 minute cylinder assay is unaffected by administration of CNO. P > 0.24 (see Supplementary Table 1), two-way ANOVA with Tukey’s multiple comparisons test, n = 6 mice from one experiment. c, Turning preference in an open field arena is unaffected by administration of CNO. P > 0.42, two-way ANOVA with Tukey’s multiple comparisons test, n = 6 mice from one experiment. Box-and-whisker plots in (c, d) give the median, 25th and 75th percentiles, and range. d, Instantaneous quantification of ipsilateral and contralateral revolutions for mice in (c) after injection of either saline or CNO in AAV-FLEX-mCherry injected mice. Time-series data are plotted as mean ± standard error mean.
Extended Data Fig. 3 Analysis of open field locomotor performance in Chx10Cre > FLEX-hM3Dq and Chx10Cre > FLEX-TeLC mice.
a-d, Open field locomotor performance in Chx10Cre > FLEX-hM3Dq mice after administration of saline or CNO. CNO administration significantly decreased the velocity of locomotor bouts (a, ***P < 0.001, paired two-tailed t-test, n = 12 mice from three independent experiments), with no effect on the distance traveled (b, P = 0.40, paired two-tailed t-test, n = 12 mice), ambulation (c, P = 0.46, paired two-tailed t-test, n = 12 mice), or number of stops per minute (d, P = 0.11, paired two-tailed t-test, n = 12 mice). e–h, Open field locomotor performance in Chx10Cre > FLEX-TeLC mice 1 day before injection versus 6 days after injection. TeLC expression significantly increased the velocity of locomotor bouts (e, *P = 0.015, paired two-tailed t-test, n = 10 mice from two independent experiments) and the distance traveled (f, *P = 0.022, paired two-tailed t-test, n = 10 mice from two independent experiments), with no effect on ambulation (g, P = 0.07, paired two-tailed t-test, n = 10 mice) or the number of stops per minute (h, P = 0.24, paired two-tailed t-test, n = 10 mice).
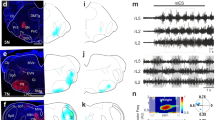
Extended Data Fig. 4 Unilateral activation of Chx10 Gi neurons causes inhibition of ipsilateral rhythmic flexor locomotor activity and prolongation of ipsilateral extensor locomotor activity.
a, Schematic of split-bath brainstem-spinal cord preparation from P0-4 Chx10Cre;R26RChR2 mice, as in Fig. 4. Recordings are taken from the ipsilateral L2 (flexor-related) and L5 (extensor-related) ventral roots. b-c, Unilateral photostimulation of Chx10 Gi neurons reduces the frequency (b) and/or the amplitude (c) of locomotor-like flexor activity ipsilateral (ipsi L2) to the stimulation (compare with Fig. 4), with a simultaneous prolongation of extensor-related burst duration (ipsi L5). Traces in (b,c) are derived from two different mice and are representative of n = 4 independent preparations. d, Integrated traces normalized in amplitude from 0–1 and averaged across trials for each mouse (n = 4 mice, grey), with the grand average across mice represented in black. e, 33 trials from 4 mice represented as intensity plots from 0–1, which are integrated traces normalized from 0–1. 3 of the mice represented for ipsi-L2 are also represented in Fig. 4.
Extended Data Fig. 5 Blocking inhibition in the spinal cord reveals an opposite effect of Chx10 Gi stimulation on axial and locomotor networks.
a, Schematic of split-bath brainstem-spinal cord preparation from P0-P4 Chx10Cre;R26RChR2 mice, as in Fig. 4. Recordings are taken from the ipsilateral thoracic (Th, axial), L2 (flexor-related), and L5 (extensor-related) ventral roots. Picrotoxin (PTX, 10 µM) was added to the caudal pool to block inhibition in the spinal cord. b, Recordings from ipsilateral Th7, L2, and L5 ventral roots in presence of locomotor promoting drugs (NMDA/5HT) demonstrating that unilateral photostimulation of Chx10 Gi neurons reduces the locomotor-like flexor activity ipsilateral (ipsi L2) to the stimulation, with a simultaneous prolongation of extensor-related burst durations (ipsi L5), and a tonic increase of thoracic activity lasting for the stimulus duration. (n = 4 independent preparations, 46 trials). c, In the presence of PTX, photostimulation of Chx10 Gi neurons no longer affected rhythmic lumbar locomotor-like activity (n = 5 independent preparations, 69 trials), whereas activation of thoracic (axial) motor activity is still present.
Extended Data Fig. 6 Stride length asymmetries versus heading position in wild-type, Chx10Cre > FLEX-hM3Dq, and Chx10Cre > FLEX-TeLC mice.
a, b, Analysis of individual steps relative to direction of movement in wild-type (WT), Chx10Cre > FLEX-hM3Dq (hM3Dq), and Chx10Cre > FLEX-TeLC (TeLC) mice. Stride length (cm) was measured on the ipsilateral and contralateral side together with the direction of movement. Stride length values are positive when the stride length is longer on the contralateral side. Positive changes in angle reflect an ipsilateral turn whereas negative values are contralateral. 6016 individual steps were analyzed from 6 wildtype (WT) (352 strides), 6 hM3Dq (477 strides), and 6 TeLC (5187 strides) mice. ***P < 0.001 for regression in (a) and (b), F-test, n = 6016 strides. c-d, Data for individual locomotor bouts (representing the average of all steps in a locomotor bout) from WT, hM3Dq, and TeLC mice. 708 locomotor bouts were analyzed from 6 WT (63 bouts), 6 hM3Dq (61 bouts), and 6 TeLC (584 bouts) mice. ***P < 0.001 for regression in (c) and (d), F-test, n = 708 locomotor bouts. Full information on regression analyses for WT, hM3Dq, TeLC, and pooled data can be found in Supplementary Table 1. Goodness of fit is given as the coefficient of determination (r2; the square of Pearson’s r).
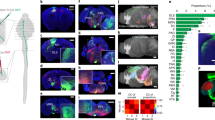
Extended Data Fig. 7 Monosynaptic rabies tracing identifies Chx10 Gi presynaptic inputs.
a, A rabies transsynaptic tracing approach was used to identify presynaptic inputs to Chx10 Gi neurons (see also, Fig. 7 and Supplementary Table 2). b, Injection site in rostral Gi. Initial site of infection is visualized as a large population of mCherry+ neurons, accompanied by dense mCherry+ processes. Starter neurons of the ipsilateral Gi do not exhibit a strong input from neurons of the contralateral Gi. c, Quantification of mCherry-labeled neurons in the ipsilateral and contralateral Gi. ***P = 0.0002, paired two-tailed t-test, n = 6 mice from one experiment. d, Bilateral input to Chx10 Gi neurons from neurons of primary motor and somatosensory cortex. Open yellow triangles point to soma from pyramidal neurons. Yellow asterisk indicates an apical dendrite. e, Quantification of mCherry-labeled neurons in the ipsilateral and contralateral cortex. ***P = 0.001, paired two-tailed t-test, n = 6 mice from one experiment. f, Input to Chx10 Gi neurons from neurons of the ipsilateral zona incerta. Presynaptic neurons were observed primarily in the dorsal (ZID) and caudal aspects of the zona incerta. g, Quantification of mCherry-labeled neurons in the ipsilateral and contralateral zona incerta. ***P < 0.001, paired two-tailed t-test, n = 6 mice from one experiment. h, Unilateral input to Chx10 Gi neurons from the ipsilateral mesencephalic reticular formation. i, Quantification of mCherry-labeled neurons in the ipsilateral and contralateral mesencephalic reticular formation. ***P = 0.001, paired two-tailed t-test, n = 6 mice from one experiment. j, Medial (Med) and lateral (Lat) deep cerebellar nuclei exhibited unilateral or bilateral, respectively, input to Chx10 Gi neurons. k, Quantification of mCherry-labeled neurons in the deep cerebellar nuclei. Med, **P = 0.0024, paired two-tailed t-test, n = 6 mice from one experiment; Lat, P = 0.69, paired two-tailed t-test, n = 6 mice from one experiment. Box-and-whisker plots in (c, e, g, i, k) give the median, 25th and 75th percentiles, and range.
Supplementary information
Supplementary Table 1
Statistics summary. Full information for statistical methods and tests employed for each dataset.
Supplementary Table 2
Summary of monosynaptic rabies tracing data.
Supplementary Video 1
Open-field analysis of a left Gi Chx10Cre > FLEX-hM3Dq-targeted mouse 0, 15 and 25 min after administration of CNO. Clip 1: immediately after CNO administration (0 min), the mouse exhibits no preference in locomotor direction. Clip 2: after 15 min, the mouse exhibits a clear preference toward the ipsilateral side, making large left-turn circles in the arena. Clip 3: after 25 min, the mouse exhibits sharp rotations toward the ipsilateral side. Playback is three times real speed. The mouse in this video represents n = 12 mice from three independent experiments. These data correspond with Fig. 2a–d.
Supplementary Video 2
Photostimulation of left Gi Chx10Cre > FLEX-ChR2-targeted mice during spontaneous locomotion and at rest. Clip 1: photostimulation of Chx10 Gi neurons induces a sharp turn toward the ipsilateral side. Clips 2–4: photostimulation of Chx10 Gi neurons induces a more gradual turn toward the ipsilateral side (clips 1–3 are examples from the same mouse, whereas clip 4 is from another mouse). Clip 5: photostimulation of Chx10 Gi neurons during rearing evokes an axial bend toward the ipsilateral side. Clip 6: photostimulation of Chx10 Gi neurons at rest evokes an axial bend toward the ipsilateral side. The four mice in these videos represent n = 7 mice from two independent experiments. These data correspond with Fig. 2e–i.
Supplementary Video 3
Open-field analysis of a left Gi Chx10Cre > FLEX-TeLC-targeted mouse. Clip 1: defore viral injection (day −1), the mouse exhibited no left or right preference in locomotor direction. Clip 2: 6 d after viral injection, the mouse exhibits a clear locomotor preference toward the contralateral (right) side. Playback is three times real speed. The mouse in this video represents n = 10 mice from two independent experiments. These data correspond with Fig. 3a–d.
Supplementary Video 4
DeepLabCut tracking of footfalls and movement trajectories in WT, Chx10Cre > FLEX-hM3Dq- and Chx10Cre > FLEX-TeLC-targeted mice. Clip 1: video of a WT mouse walking straight. Clip 2: video of a WT mouse taking a spontaneous right turn (clips 1 and 2 represent n = 6 WT mice). Clip 3: video of a Chx10Cre > FLEX-hM3Dq (left Gi)-targeted mouse taking an ipsilateral turn (representing n = 6 Chx10Cre > FLEX-hM3Dq mice from two independent experiments). Clip 4: video of a Chx10Cre > FLEX-TeLC (left Gi)-targeted mouse taking a contralateral turn (representing n = 6 Chx10Cre > FLEX-TeLC mice from two independent experiments). The footfalls are plotted when the paw velocity reaches 0 cm −1. These data correspond with Fig. 5 and Extended Data Fig. 6.
Rights and permissions
About this article
Cite this article
Cregg, J.M., Leiras, R., Montalant, A. et al. Brainstem neurons that command mammalian locomotor asymmetries. Nat Neurosci 23, 730–740 (2020). https://doi.org/10.1038/s41593-020-0633-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-020-0633-7