Abstract
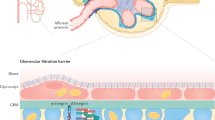
Mammalian kidneys constantly filter large amounts of liquid, with almost complete retention of albumin and other macromolecules in the plasma. Breakdown of the three-layered renal filtration barrier results in loss of albumin into urine (albuminuria) across the wall of small renal capillaries, and is a leading cause of chronic kidney disease. However, exactly how the renal filter works and why its permeability is altered in kidney diseases is poorly understood. Here we show that the permeability of the renal filter is modulated through compression of the capillary wall. We collect morphometric data prior to and after onset of albuminuria in a mouse model equivalent to a human genetic disease affecting the renal filtration barrier. Combining quantitative analyses with mathematical modelling, we demonstrate that morphological alterations of the glomerular filtration barrier lead to reduced compressive forces that counteract filtration pressure, thereby resulting in capillary dilatation, and ultimately albuminuria. Our results reveal distinct functions of the different layers of the filtration barrier and expand the molecular understanding of defective renal filtration in chronic kidney disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Assady, S., Benzing, T., Kretzler, M. & Skorecki, K. L. Glomerular podocytes in kidney health and disease. Lancet Lond. Engl. 393, 856–858 (2019).
Cheng, C.-H. Albuminuria in childhood is a risk factor for chronic kidney disease and end-stage renal disease. Pediatr. Neonatol. 57, 263–264 (2016).
Gerstein, H. C. et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 286, 421–426 (2001).
Haraldsson, B., Nyström, J. & Deen, W. M. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol. Rev. 88, 451–487 (2008).
Gerke, P. et al. NEPH2 is located at the glomerular slit diaphragm, interacts with nephrin and is cleaved from podocytes by metalloproteinases. J. Am. Soc. Nephrol. 16, 1693–1702 (2005).
Perico, L., Conti, S., Benigni, A. & Remuzzi, G. Podocyte–actin dynamics in health and disease. Nat. Rev. Nephrol. 12, 692–710 (2016).
Pavenstädt, H., Kriz, W. & Kretzler, M. Cell biology of the glomerular podocyte. Physiol. Rev. 83, 253–307 (2003).
Deen, W. M. What determines glomerular capillary permeability? J. Clin. Invest. 114, 1412–1414 (2004).
Jeansson, M. & Haraldsson, B. Glomerular size and charge selectivity in the mouse after exposure to glucosaminoglycan-degrading enzymes. J. Am. Soc. Nephrol. 14, 1756–1765 (2003).
Bartlett, C. S., Jeansson, M. & Quaggin, S. E. Vascular growth factors and glomerular disease. Annu. Rev. Physiol. 78, 437–461 (2016).
Dimke, H., Maezawa, Y. & Quaggin, S. E. Crosstalk in glomerular injury and repair. Curr. Opin. Nephrol. Hypertens. 24, 231–238 (2015).
Jin, J. et al. Soluble FLT1 binds lipid microdomains in podocytes to control cell morphology and glomerular barrier function. Cell 151, 384–399 (2012).
Caulfield, J. P. & Farquhar, M. G. The permeability of glomerular capillaries to graded dextrans. Identification of the basement membrane as the primary filtration barrier. J. Cell Biol. 63, 883–903 (1974).
Barker, D. F. et al. Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science 248, 1224–1227 (1990).
Zenker, M. et al. Human laminin β2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum. Mol. Genet. 13, 2625–2632 (2004).
Matejas, V. et al. Mutations in the human laminin β2 (LAMB2) gene and the associated phenotypic spectrum. Hum. Mutat. 31, 992–1002 (2010).
Brinkkoetter, P. T., Ising, C. & Benzing, T. The role of the podocyte in albumin filtration. Nat. Rev. Nephrol. 9, 328 (2013).
Akchurin, O. & Reidy, K. J. Genetic causes of proteinuria and nephrotic syndrome: impact on podocyte pathobiology. Pediatr. Nephrol. 30, 221–233 (2015).
Preston, R., Stuart, H. M. & Lennon, R. Genetic testing in steroid-resistant nephrotic syndrome: why, who, when and how? Pediatr. Nephrol. 34, 195–210 (2019).
Hermle, T., Braun, D. A., Helmstädter, M., Huber, T. B. & Hildebrandt, F. Modeling monogenic human nephrotic syndrome in the Drosophila garland cell nephrocyte. J. Am. Soc. Nephrol. 28, 1521–1533 (2017).
Lovric, S., Ashraf, S., Tan, W. & Hildebrandt, F. Genetic testing in steroid-resistant nephrotic syndrome: when and how? Nephrol. Dial. Transplant. 31, 1802–1813 (2016).
Hildebrandt, F. & Heeringa, S. F. Specific podocin mutations determine age of onset of nephrotic syndrome all the way into adult life. Kidney Int. 75, 669–671 (2009).
Mikó, Á., K Menyhárd, D., Kaposi, A., Antignac, C. & Tory, K. The mutation-dependent pathogenicity of NPHS2 p.R229Q: a guide for clinical assessment. Hum. Mutat. 39, 1854–1860 (2018).
Tory, K. et al. Mutation-dependent recessive inheritance of NPHS2-associated steroid-resistant nephrotic syndrome. Nat. Genet. 46, 299–304 (2014).
Artelt, N. et al. Comparative analysis of podocyte foot process morphology in three species by 3D super-resolution microscopy. Front. Med. 5, 292 (2018).
Ichimura, K. et al. Morphological processes of foot process effacement in puromycin aminonucleoside nephrosis revealed by FIB/SEM tomography. J. Am. Soc. Nephrol. 30, 96 (2019).
Suleiman, H. Y. et al. Injury-induced actin cytoskeleton reorganization in podocytes revealed by super-resolution microscopy. JCI Insight 2, 94137 (2017).
Lechner, M. S. & Dressler, G. R. The molecular basis of embryonic kidney development. Mech. Dev. 62, 105–120 (1997).
Wharram, B. L. et al. Podocyte depletion causes glomerulosclerosis: diphtheria toxin–induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J. Am. Soc. Nephrol. 16, 2941 (2005).
Kim, Y. H. et al. Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int. 60, 957–968 (2001).
Rinschen, M. M. et al. N-degradomic analysis reveals a proteolytic network processing the podocyte cytoskeleton. J. Am. Soc. Nephrol. JASN 28, 2867–2878 (2017).
Rinschen, M. M. et al. A multi-layered quantitative in vivo expression atlas of the podocyte unravels kidney disease candidate genes. Cell Rep. 23, 2495–2508 (2018).
Suh, J. H. & Miner, J. H. The glomerular basement membrane as a barrier to albumin. Nat. Rev. Nephrol. 9, 470–477 (2013).
Unnersjö-Jess, D., Scott, L., Blom, H. & Brismar, H. Super-resolution stimulated emission depletion imaging of slit diaphragm proteins in optically cleared kidney tissue. Kidney Int. 89, 243–247 (2016).
Huber, T. B., Kottgen, M., Schilling, B., Walz, G. & Benzing, T. Interaction with podocin facilitates nephrin signaling. J. Biol. Chem. 276, 41543–41546 (2001).
Smithies, O. Why the kidney glomerulus does not clog: a gel permeation/diffusion hypothesis of renal function. Proc. Natl Acad. Sci. USA 100, 4108–4113 (2003).
Deen, W., Robertson, C. & Brenner, B. A model of glomerular ultrafiltration in the rat. Am. J. Physiol. 223, 1178–1183 (1972).
Daniels, B. S., Hauser, E. B., Deen, W. M. & Hostetter, T. H. Glomerular basement membrane: in vitro studies of water and protein permeability. Am. J. Physiol. 262, F919–F926 (1992).
Deen, W. M., Lazzara, M. J. & Myers, B. D. Structural determinants of glomerular permeability. Am. J. Physiol. Renal Physiol. 281, F579–F596 (2001).
Fissell, W. H. & Miner, J. H. What is the glomerular ultrafiltration barrier? J. Am. Soc. Nephrol. 29, 2262–2264 (2018).
Robinson, G. B. & Walton, H. A. Glomerular basement membrane as a compressible ultrafilter. Microvasc. Res. 38, 36–48 (1989).
Kriz, W. et al. A role for podocytes to counteract capillary wall distension. Kidney Int. 45, 369–376 (1994).
Neal, C. R., Crook, H., Bell, E., Harper, S. J. & Bates, D. O. Three-dimensional reconstruction of glomeruli by electron microscopy reveals a distinct restrictive urinary subpodocyte space. J. Am. Soc. Nephrol. 16, 1223–1235 (2005).
Lazzara, M. J. & Deen, W. M. Model of albumin reabsorption in the proximal tubule. Am. J. Physiol. Ren. Physiol. 292, F430–F439 (2007).
Dickson, L. E., Wagner, M. C., Sandoval, R. M. & Molitoris, B. A. The proximal tubule and albuminuria: really! J. Am. Soc. Nephrol. 25, 443–453 (2014).
Tsai, P. S. et al. Correlations of neuronal and microvascular densities in murine cortex revealed by direct counting and colocalization of nuclei and vessels. J. Neurosci. 29, 14553 (2009).
Hall, J. E., Guyton, A. C., Smith, M. J. & Coleman, T. G. Blood pressure and renal function during chronic changes in sodium intake: role of angiotensin. Am. J. Physiol. 239, F271–F280 (1980).
Heller, J., Horácek, V. & Angiotensin, I. I. Preferential efferent constriction? Ren. Physiol. 9, 357–365 (1986).
Reiser, J. et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat. Genet. 37, 739–744 (2005).
Moeller, M. J. & Tenten, V. Renal albumin filtration: alternative models to the standard physical barriers. Nat. Rev. Nephrol. 9, 266–277 (2013).
Russo, L. M. et al. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney Int. 71, 504–513 (2007).
Lund, U. et al. Glomerular filtration rate dependence of sieving of albumin and some neutral proteins in rat kidneys. Am. J. Physiol. Ren. Physiol. 284, F1226–F1234 (2003).
Chang, R. L. et al. Permselectivity of the glomerular capillary wall to macromolecules. II. Experimental studies in rats using neutral dextran. Biophys. J. 15, 887–906 (1975).
Schmieder, S., Nagai, M., Orlando, R. A., Takeda, T. & Farquhar, M. G. Podocalyxin activates RhoA and induces actin reorganization through NHERF1 and ezrin in MDCK cells. J. Am. Soc. Nephrol. 15, 2289–2298 (2004).
Takeda, T., McQuistan, T., Orlando, R. A. & Farquhar, M. G. Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J. Clin. Invest. 108, 289–301 (2001).
Kriz, W. & Lemley, K. V. Mechanical challenges to the glomerular filtration barrier: adaptations and pathway to sclerosis. Pediatr. Nephrol. Berl. Ger. 32, 405–417 (2017).
Lawrence, M. G. et al. Permeation of macromolecules into the renal glomerular basement membrane and capture by the tubules. Proc. Natl Acad. Sci. USA 114, 2958–2963 (2017).
Fujigaki, Y. et al. Intra-GBM site of the functional filtration barrier for endogenous proteins in rats. Kidney Int. 43, 567–574 (1993).
Hausmann, R. et al. Electrical forces determine glomerular permeability. J. Am. Soc. Nephrol. JASN 21, 2053–2058 (2010).
Höhne, M. et al. Single-nephron proteomes connect morphology and function in proteinuric kidney disease. Kidney Int. 93, 1308–1319 (2018).
Wang, H. et al. One-Step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153, 910–918 (2013).
Tröder, S. E. et al. An optimized electroporation approach for efficient CRISPR/Cas9 genome editing in murine zygotes. PLoS ONE 13, e0196891 (2018).
Rinschen, M. M. et al. Phosphoproteomic analysis reveals regulatory mechanisms at the kidney filtration barrier. J. Am. Soc. Nephrol. 25, 1509–1522 (2014).
Hughes, C. S. et al. Ultrasensitive proteome analysis using paramagnetic bead technology. Mol. Syst. Biol. 10, 757 (2014).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Steger, C. An unbiased detector of curvilinear structures. IEEE Trans. Pattern Anal. Mach. Intell. 20, 113–125 (1998).
Grill, A. et al. Salt-losing nephropathy in mice with a null mutation of the Clcnk2 gene. Acta Physiol. Oxf. Engl. 218, 198–211 (2016).
Chen, L. et al. Regulation of renin in mice with Cre recombinase-mediated deletion of G protein Gsα in juxtaglomerular cells. Am. J. Physiol. Ren. Physiol. 292, F27–F37 (2007).
Acknowledgements
We thank M. Brütting, R. Herzog, S. Keller and S. Kayser for excellent technical support. We thank J. Patrakka (KI/AZ Integrated CardioMetabolic Center, Department of Laboratory Medicin,. Karolinska Institutet at Karolinska University Hospital Huddinge, Stockholm, Sweden) for providing us with kidney tissue of a tumour nephrectomy. The plasmid pSpCas9(BB)‐2A‐GFP (PX458) was a gift from F. Zhang (Addgene plasmid no. 48138). This work was supported by the Clinical Research Unit (CRU) 329 (KFO 329; A1, A6 and A7) as well as partly by FOR 2743 of the Deutsche Forschungsgemeinschaft (DFG).
Author information
Authors and Affiliations
Contributions
Conceptualization, B.S. and T.B.; methodology, L.B., L.K.E., M. Höhne, B.S. and T.B.; software, L.B. and M. Höhne; formal analysis, L.B., M. Höhne, A.E., V.Z., K.F. and M.M.R.; investigation, L.B., D.U.-J., M. Höhne, J.B.-L., D.R., R.H., V.Z., K.F. and M. Helmstädter.; resources, M.C.L., K.T, B.B.B. and P.F.H..; writing, original draft, L.B., M. Höhne, B.S. and T.B.; writing, review and editing, L.B., D.U.-J., M. Höhne., A.E., M.M.R., L.K.E., H.C., M.J.H., G.W., P.T.B., M.C.L., B.S. and T.B.; supervision, M. Höhne, H.C., M.J.H., G.W., P.T.B., H. Brismar, H. Blom, B.S. and T.B.; project administration, B.S. and T.B.; funding acquisition, P.T.B., B.S. and T.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Primary Handling Editor: Pooja Jha.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Sanger sequencing of F1 generation mice demonstrates heterozygosity.
a, The upper panel indicates the wildtype sequences on the gDNA and amino acid levels of the region neighboring the R231Q mutation site. The wildtype nucleotide at position c.692 and the wildtype amino acid at position p.231 are highlighted in green. Sequencing of a wildtype mouse aligns with the predicted wildtype sequence. The lower panel indicates the R231Q sequences on the gDNA and amino acid levels of the same region. The point mutation at c.692 and the respective change in the amino acid at position p.231 are highlighted in red. Silent mutations leading to restriction sites are highlighted in orange. Sequencing of a F1 mouse reveals double signals at the respective mutation sites. The black arrow indicates the point mutation and asterisks indicate silent mutation sites. b, The upper panel indicates the wildtype sequences on the gDNA and amino acid levels of the region neighboring the A286V mutation site. The wildtype nucleotide at position c.857 and the wildtype amino acid at position p.286 are highlighted in green. Sequencing of a wildtype mouse align with the predicted wildtype sequence. The lower panel indicates the A286V sequences on the gDNA and amino acid levels of the same region. The point mutation at c.857 and the respective change in the amino acid at position p.286 are highlighted in red. Sequencing of a F1 mouse reveals a double signal at the point mutation site indicated by the black arrow.
Extended Data Fig. 2 Glomerular proteome of wildtype and PodR231Q/A286V mice at 3 weeks of age.
a, Hierarchical clustering of proteins (label-free quantification (LFQ) values) within 3 control and 3 PodR231Q/A286V mice samples based on Euclidian distance. Heat map displays normalized log2 LFQ intensities of all n = 4322 proteins quantified in the samples (red = high intensity, green = low intensity). b, Volcano plot showing logarithmized fold changes of label-free quantification (LFQ) values in control vs. PodR231Q/A286V samples. Log2 ratios of PodR231Q/A286V over control are plotted against the negative logarithmic P value of the two-sided Student’s t-test. Each dot represents a protein (FDR = 0.05, s0 = 0.1). Proteins above the curved line are considered significantly different in terms of abundance. c, The same Volcano plot as in b with podocyte-specific proteins highlighted in red. d, The same Volcano plot as in b with glomerular basement membrane constituents highlighted in green.
Extended Data Fig. 3 Pedigree and clinical history of a patient suffering from PodR229Q/A284V mutations.
a, Schematic drawings of the human podocin protein with the two respective point mutations. Highlighted in yellow and red are the transmembrane domain (TM) and the Prohibitin domain (PHB). The pedigree indicates the mode of inheritance in the patient’s family. The parents as well as the sibling are unaffected carriers of one of the point mutations. b, Measured serum creatinine levels at different ages of the PodR229Q/A284V patient. c, Urinary protein to creatinine ratios at different ages of the PodR229Q/A284V patient. The patient was coincidentally diagnosed with albuminuria at 4 years of age. d, Normalized creatinine clearances at different ages of the PodR229Q/A284V patient shows a decline in renal function in adolescence/early adulthood.
Extended Data Fig. 4 Electron micrographs reveal progressive pathological alterations of the podocytes in PodR231Q/A286V mice.
a, Scanning electron microscopy (SEM) of control mice at four different time points shows regularly configured podocytes with typical interdigitating pattern of foot processes. Scale bars correspond to 5 µm. b, SEM of PodR231Q/A286V mice at different time points detects a progressive widening of foot processes and a rarification of the interdigitating pattern. Scale bars correspond to 5 μm. c, Transmission electron microscopy (TEM) of control mice at different time points reveals complete coverage of the glomerular basement membrane (GBM) by the podocyte’s foot processes and cell bodies (CB) of the podocytes that reside within the bowman’s space (BS) (upper panel). Higher magnification in lower panel allows for the visualization of the slit diaphragm (yellow arrows) and the slender foot processes (yellow dotted line). Scale bars correspond to 500 nm (upper panels) and 250 nm (lower panels). d, TEM of PodR231Q/A286V mice at different time points detects podocyte’s cell bodies (CB) directly attached to the glomerular basement membrane (light yellow line), loss of the slit diaphragm (yellow asterisks), and irregular thickening of the glomerular basement membrane at later time points (yellow arrowhead). Higher magnification (lower panel) demonstrates widening of foot processes (yellow dotted line), and ultimately, absence of foot processes (light yellow area). One mouse per age and genotype was imaged. Scale bars correspond to 500 nm (upper panels) and 250 nm (lower panels).
Extended Data Fig. 5 Quantification of morphometric parameters.
a, Workflow of the quantification of foot process (FP) area, perimeter, and circularity. b, Workflow of the quantification of the slit diaphragm (SD) length per area. Data in (a) are shown as mean ± SD. The depicted values in (a) represent measurements from 3 images each of one mouse per genotype c, Workflow of the quantification of the slit diaphragm (SD) grid index. d, Demonstration of the effect of different manual assignment of the capillary surface on the slit diaphragm (SD) length per area and slit diaphragm (SD) grid index values. The difference in the manual assignment does affect the SD length whereas the values of the SD grid index remain very similar. e, Scatter plot of the values of slit diaphragm (SD) grid index against the values of slit diaphragm (SD) length per area of all experimental mice (control and PodR231Q/A286V). Based on Spearman’s rank coefficient (r), there is strong correlation between slit diaphragm (SD) grid index and slit diaphragm (SD) length per area. Each dot represents an individual mouse of which both parameters were determined (n = 43, control and PodR231Q/A286V mice). r, Spearman’s rank coefficient; P-value corresponds to Spearman’s correlation.
Extended Data Fig. 6 Morphological parameters correlate with albuminuria across the entire group of experimental mice (PodR231Q/A286V and control mice).
(a)-(g) Scatter plots of each indicated parameter against the urinary albumin creatinine ratio (ACR) of experimental mice. Across the entire group of experimental mice all parameters with the exception of the foot process (FP) area (p = 0.051) correlate significantly with the ACR. (a)-(b) n = 19 mice, (c)-(g) n = 27 mice. Two-tailed Spearman’s rank correlation coefficient was used to determine statistical significance. r, Spearman’s rank coefficient. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
Extended Data Fig. 7 Glomerular filtration rate (GFR) of control (black circles) and PodR231Q/A286V (red triangles) mice at different time points and correlation of the GFR with slit diaphragm (SD) length and urinary albumin creatinine ratio (ACR).
a-b, Measurement of the GFR at the indicated time points. Values are depicted as absolute (a) and normalized to body weight (b). Each dot/triangle represents one mouse (n ≥ 5 mice per genotype and age). c-d, Scatter plots of the normalized GFR against the slit diaphragm (SD) length per area (c) and the ACR of individual experimental mice (d). There is no significant correlation between the GFR and the SD length or the ACR within the groups of control and PodR231Q/A286V mice. Each dot/triangle represents one mouse. Data are presented as mean± SEM. Two-tailed unpaired Student’s t-tests and Spearman rank coefficients were used to determine statistical significance. r, Spearman’s rank coefficient. * p < 0.05, ** p < 0.01.
Supplementary information
Supplementary Information
Supplementary Methods and Table 1
Rights and permissions
About this article
Cite this article
Butt, L., Unnersjö-Jess, D., Höhne, M. et al. A molecular mechanism explaining albuminuria in kidney disease. Nat Metab 2, 461–474 (2020). https://doi.org/10.1038/s42255-020-0204-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-020-0204-y
This article is cited by
-
The podocytes’ inflammatory responses in experimental GN are independent of canonical MYD88-dependent toll-like receptor signaling
Scientific Reports (2024)
-
Role of biophysics and mechanobiology in podocyte physiology
Nature Reviews Nephrology (2024)
-
The proteasome modulates endocytosis specifically in glomerular cells to promote kidney filtration
Nature Communications (2024)
-
Renal clearance of graphene oxide: glomerular filtration or tubular secretion and selective kidney injury association with its lateral dimension
Journal of Nanobiotechnology (2023)
-
In vivo characterization of a podocyte-expressed short podocin isoform
BMC Nephrology (2023)