Abstract
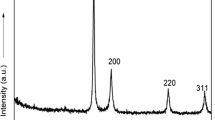
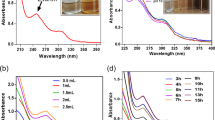
Functional group protections are the key to the synthesis of targeted molecules. In particular, the acetylation reaction is considered as one of the important organic transformations for the preparation of useful products, such as polymers, food additives, cosmetics, medicines and perfumes. Unfortunately, most these methods employed for the acetylation process encompassed the use of acids, bases and hazardous reagents. In this manuscript, a simple and green synthesis of silver nanoparticles (AgNPs) is described using water extract of onion peel as reductant. The synthesized AgNPs were then employed as green catalyst in the acetylation of alcohols and amines. In the transmission electron microscopy analysis, the AgNPs were successfully prepared with an average size of 12.5 nm and was found to be in spherical size without any agglomeration being observed. Moreover, the synthesized AgNPs showed remarkable catalytic activity by mediating the synthesis of various acetates and amides in good to excellent yields (72–95% yields). In addition, the synthesized AgNPs are recyclable and can be utilized up to five times for the subsequent reactions, without significant lost in catalytic efficiency (93–95%). The current method offers many benefits such as environmental benign and green chemical process compared to previous works.








Similar content being viewed by others
References
Das, R.; Chakraborty, D.: Silver triflate catalyzed acetylation of alcohols, thiols, phenols, and amines. Synthesis 2011, 1621–1625 (2011)
Scriven, E.F.: 4-Dialkylaminespyridines: super acylation and alkylation catalysts. Chem. Soc. Rev. 12, 129–161 (1983)
Shaik, M.; Ali, Z.; Khan, M.; Kuniyil, M.; Assal, M.E.; Alkhathlan, H.Z.; Al-Warthan, A.; Siddiqui, M.R.; Khan, M.; Adil, S.F.: Green synthesis and characterization of palladium nanoparticles using Origanum vulgare L. extract and their catalytic activity. Molecules 22, 165 (2017)
Mojtahedi, M.M.; Samadian, S.: Efficient and rapid solvent-free acetylation of alcohols, phenols, and thiols using catalytic amounts of sodium acetate trihydrate. J. Chem. 2013, 1–7 (2013)
Prajapti, S.K.; Nagarsenkar, A.; Babu, B.N.: Tris(pentafluorophenyl) borane catalyzed acylation of alcohols, phenols, amines, and thiophenols under solvent-free condition. Tetrahedron Lett. 55, 1784–1787 (2014)
Alam, M.; Rahman, A.; Alandis, N.M.; Shaik, M.R.: Ni/Silica catalysed acetylation of phenols and naphthols: an eco-friendly approach. Arab. J. Chem. 7, 53–56 (2014)
Kumar, N.U.; Reddy, B.S.; Reddy, V.P.; Bandichhor, R.: Zinc triflate catalyzed acylation of alcohols, phenols, and thiophenols. Tetrahedron Lett. 55, 910–912 (2014)
Joo, S.R.; Youn, Y.J.; Hwang, Y.R.; Kim, S.H.: Highly active manganese mediated acylation of alcohols with acid chlorides or anhydrides. Synlett 28, 2665–2669 (2017)
Bajracharya, G.B.; Shrestha, S.S.: Unprecedented acetylation of phenols using a catalytic amount of magnesium powder. Synth. Commun. 48, 1688–1693 (2018)
Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K.: Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J. Nanotechnol. 9, 1050–1074 (2018)
Bharathi, D.; Diviya, Josebin M.; Vasantharaj, S.; Bhuvaneshwari, V.: Biosynthesis of silver nanoparticles using stem bark extracts of Diospyros montana and their antioxidant and antibacterial activities. J. Nanostruct. Chem. 8, 83–92 (2018)
Dong, X.Y.; Gao, Z.W.; Yang, K.F.; Zhang, W.Q.; Xu, L.W.: Nanosilver as a new generation of silver catalysts in organic transformations for efficient synthesis of fine chemicals. Catal. Sci. Technol. 5, 2554–2574 (2015)
Ahlawat, D.S.; Kumari, R.; Rachna, Y.I.: Synthesis and characterization of sol–gel prepared silver nanoparticles. Int. J. Nanosci. 13, 1–7 (2014)
Adner, D.; Noll, J.; Schulze, S.; Hietschold, M.; Lang, H.: Asperical silver nanoparticles by thermal decomposition of a single-source-precursor. Inorganica Chim. Acta 446, 19–23 (2016)
Alagumuthu, G.; Kirubha, R.: Synthesis and characterisation of silver nanoparticles in different medium. Open. J. Synth. Theory Appl. 1, 13–17 (2012)
Awad, M.A.; Hendi, A.A.; Ortashi, K.M.; Alotaibi, R.A.; Sharafeldin, M.S.: Characterization of silver nanoparticles prepared by wet chemical method and their antibacterial and cytotoxicity activities. Trop. J. Pharm. Res. 15, 679–685 (2016)
Hussain, I.; Singh, N.B.; Singh, A.; Singh, H.; Singh, S.C.: Green synthesis of nanoparticles and its potential application. Biotechnol. Lett. 38, 545–560 (2016)
Butola, B.S.; Gupta, A.; Roy, A.: Multifunctional finishing of cellulosic fabric via facile, rapid in situ green synthesis of AgNPs using pomegranate peel extract biomolecules. Sustain. Chem. Pharm. 12, 100135 (2019)
Omole, R.K.; Torimiro, N.; Alayande, S.O.; Ajenifuja, E.: Silver nanoparticles synthesized from Bacillus subtilis for detection of deterioration in the post-harvest spoilage of fruit. Sustain. Chem. Pharm. 10, 33–40 (2018)
Zayadi, R.A.; Bakar, F.A.; Ahmad, M.K.: Elucidation of synergistic effect of eucalyptus globulus honey and Zingiber officinale in the synthesis of colloidal biogenic gold nanoparticles with antioxidant and catalytic properties. Sustain. Chem. Pharm. 13, 100156 (2019)
Zeebaree, S.Y.S.; Zeebaree, A.Y.S.: Synthesis of copper nanoparticles as oxidising catalysts for multi-component reactions for synthesis of 1, 3, 4-thiadiazole derivatives at ambient temperature. Sustain. Chem. Pharm. 13, 100155 (2019)
Parveen, K.; Banse, V.; Ledwani, L.: Green synthesis of nanoparticles: their advantages and disadvantages. AIP Conf. Proc. 1724, 1–5 (2016)
Ajitha, B.; Reddy, Y.A.K.; Reddy, P.S.: Green synthesis and characterization of silver nanoparticles using Lantana camara leaf extract. Mater. Sci. Eng., C 49, 373–381 (2015)
Behravan, M.; Panahi, A.H.; Naghizadeh, A.; Ziaee, M.; Mahdavi, R.; Mirzapour, A.: Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int. J. Biol. Macromol. 124, 148–154 (2018)
Moodley, J.S.; Krishna, S.B.N.; Pillay, K.; Govender, P.: Green synthesis of silver nanoparticles from Moringa oleifera leaf extracts and its antimicrobial potential. Adv. Nat. Sci. Nanosci. Nanotechnol. 9, 1–9 (2018)
Rauwel, P.; Küünal, S.; Ferdov, S.; Rauwel, E.: A Review on the green synthesis of silver nanoparticles and their morphologies studied via TEM. Adv. Mater. Sci. Eng. 2015, 1–9 (2015)
Chia, P.W.; Lim, B.S.; Yong, F.S.J.; Poh, S.C.; Kan, S.Y.: An efficient synthesis of bisenols in water extract of waste onion peel ash. Environ. Chem. Lett. 16, 1493–1499 (2018)
Asseri, S.N.A.R.M.; Tan, S.H.; Mohamad, W.N.K.W.; Poh, S.C.; Chia, P.W.; Kan, S.Y.; Chuah, T.S.: MgCl2 as efficient and inexpensive catalyst for the synthesis of 1, 4-dihydropyridine derivatives. Malaysian J. Anal. Sci. 21, 13–19 (2017)
Tan, S.H.; Chuah, T.S.; Chia, P.W.: An improved protocol on the synthesis of thiazolo [3, 2-a] pyrimidine using ultrasonic probe irradiation. J. Korean Chem. Soc. 60, 245–250 (2016)
Chia, P.W.; Lim, B.S.; Tan, K.C.; Yong, F.S.J.; Kan, S.Y.: Water extract of onion peel for the synthesis of bisindolylmethanes. J. King. Saud. Univ. Sci. 31, 642–647 (2019)
Ruslan, N.A.A.A.; Suk, V.R.E.; Misran, M.; Chia, P.W.: Highly efficient and green approach of synthesizing carboxylic acids from aldehydes using sodium hexametaphosphate. Sustain. Chem. Pharm. 16, 100246 (2020)
Pucciarini, L.; Ianni, F.; Petesse, V.; Pellati, F.; Brighenti, V.; Volpi, C.; Gargaro, M.; Natalini, B.; Clementi, C.; Sardella, R.: Onion (Allium cepa L.) skin: a rich resource of biomolecules for the sustainable production of colored biofunctional textiles. Molecules 24, 634 (2019)
Bhuyan, R.; Saikia, C.N.: Isolation of colour components from native dye-bearing plants in northeastern India. Bioresour. Technol. 96, 363–372 (2005)
Lu, X.; Wang, J.; Al-Qadiri, H.M.; Rose, C.F.; Powers, J.R.; Tang, J.; Rasco, B.A.: Determination of total phenolic content and antioxidant capacity of onion (Allium cepa) and shallot (Allium oschaninii) using infrared spectroscopy. Food Chem. 129, 637–644 (2011)
Mata, Y.N.; Torres, E.; Blázquez, M.L.; Baester, A.; González, F.M.J.A.; Munoz, J.A.: Gold(III) biosorption and bioreduction with the brown alga Fucus vesiculosus. J. Hazard. Mater. 166, 612–618 (2009)
Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M.: Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteau reagent. Method Enzymol. 299, 152–178 (1999)
Prior, R.L.; Wu, X.; Schaich, K.: Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary Supplements. J. Agric. Food Chem. 53, 4290–4302 (2005)
Venugopal, K.; Rather, H.A.; Rajagopal, K.; Shanthi, M.P.; Sheriff, K.; Illiyas, M.; Rather, R.A.; Manikandan, E.; Uvarajan, S.; Bhaskar, M.; Maaza, M.: Synthesis of silver nanoparticles (AgNPs) for anticancer activities (MCF breast and A549 lung cell lines) of the crude extract of Syzygium aromaticum. J. Photochem. Photobiol., B 167, 282–289 (2017)
Gnanajobitha, G.; Paulkumar, K.; Vanaja, M.; Rajeshkumar, S.; Malarkodi, C.; Annadurai, G.; Kannan, C.: Fruit-mediated synthesis of silver nanoparticles using Vitis vinifera and evaluation of their antimicrobial efficacy. J. Nanostruct. Chem. 3, 67 (2013)
Mie, R.; Samsudin, M.W.; Din, L.B.; Ahamd, A.; Ibrahim, N.; Adnan, S.N.A.: Synthesis of silver nanoparticles with antibacterial activity using the lichen Parmotrema praesorediosum. Int. J. Nanomed. 9, 121–127 (2013)
Raj, S.; Chand Mali, S.; Trivedi, R.: Green synthesis and characterization of silver nanoparticles using Enicostemma axillare (Lam.) leaf extract. Biochem. Biophys. Res. Commun. 503, 2814–2819 (2018)
Qayum, M.A.; Kumar, N.U.; Basha, M.A.; Srinivas, P.: Ferric triflate Fe(OTf)3 as a highly efficient catalyst for the acetylation of alcohols, phenols, thiols and amines: reaction mechanism understanding through density functional theory. Chem. Biol. Interf. 4, 263–267 (2014)
Kalla, R.M.N.; Reddy, S.S.; Kim, I.: Acylation of phenols, alcohols, thiols, amines and aldehydes using sulfonic acid functionalized hyper-cross-linked poly (2-naphthol) as a solid acid catalyst. Catal. Lett. 149, 2696–2705 (2019)
Khaligh, N.G.; Mihankhah, T.; Johan, M.R.; Juan, J.C.: 4-Imidazol-1-yl-butane-1- sulfonic acid ionic liquid: synthesis, structural analysis, physical properties and catalytic application as dual solvent-catalyst. Phosphorus, Sulfur Silicon Relat. Elem. 194, 866–878 (2019)
Veisi, H.; Nikseresht, A.; Rostami, A.; Hemmati, S.: Fe3O4@PEG core/shell nanoparticles as magnetic nanocatalyst for acetylation of amines and alcohols using ultrasound irradiations under solvent-free conditions. Res. Chem. Intermed. 45, 507–520 (2019)
Qayum, M.A.; Kumar, N.U.; Basha, M.A.; Srinivas, P.: Ferric triflate Fe(OTf)3 as a highly efficient catalyst for the acetylation of alcohols, phenols, thiols and amines Reaction mechanism understanding through density functional theory. Chem. Biol. Interface 4, 263–267 (2014)
Acknowledgements
The authors acknowledge the Ministry of Education Malaysia (KPM) for the Fundamental Research Grant Scheme (FRGS) (Vot. No. 59499), UMT/ RMIC/FRGS/1/2018/59499(1) and Universiti Malaysia Terengganu for providing necessary facilities to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yap, Y.H., Azmi, A.A., Mohd, N.K. et al. Green Synthesis of Silver Nanoparticle Using Water Extract of Onion Peel and Application in the Acetylation Reaction. Arab J Sci Eng 45, 4797–4807 (2020). https://doi.org/10.1007/s13369-020-04595-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-020-04595-3