Abstract
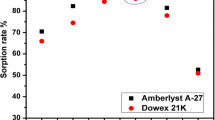
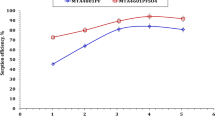
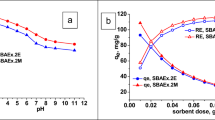
Quinoline Silicate Lewatit Composite and activated Lewatit were prepared and tested for uranium removal from sulfate solution. Uranium sorption capabilities of the tested adsorbents was estimated under different conditions; uranium initial concentration, pH, contact time, temperature, adsorbent dose and interfering ions. Experimental data obeyed Langmuir isotherm model with 69.44 mg/g and 217.39 mg/g theoretical capacity for AL and QSLC, respectively. Thermodynamic studies indicated an exothermic behavior with a decrease in randomness. Kinetics studies showed that the adsorption process obeyed pseudo-second order model. Optimum conditions were carried out for uranium recovery from a rock sample, producing uranium concentrate with 93.33% purity.
















Similar content being viewed by others
References
Dong W, Brooks SC (2008) Formation of aqueous MgUO2(CO3)2−3 complex and uranium anion exchange mechanism onto an exchange resin. Environ Sci Technol 42:1979–1983. https://doi.org/10.1021/es0711563
Shavinskii BM (2003) Anion-exchange recovery of thorium from uranium: analytical and preparation aspects. Radiochemistry 45:146–148. https://doi.org/10.1023/A:1023877008197
Wen Z, Huang K, Niu Y et al (2020) Kinetic study of ultrasonic-assisted uranium adsorption by anion exchange resin. Colloids Surf A 585:124021. https://doi.org/10.1016/j.colsurfa.2019.124021
Wódkiewicz L, Dybczyński R (1972) Effect of resin cross-linking on the anion-exchange separation of rare earth complexes with DCTA. J Chromatogr A 68:131–141. https://doi.org/10.1016/S0021-9673(00)88770-1
Rychkov VN, Smirnov AL, Gortsunova KR (2014) Sorption of uranium from underground leaching solutions with highly basic anion exchangers. Radiochemistry 56:38–42. https://doi.org/10.1134/S1066362214010081
Stoliker DL, Kaviani N, Kent DB, Davis JA (2013) Evaluating ion exchange resin efficiency and oxidative capacity for the separation of uranium(IV) and uranium(VI). Geochem Trans 14:1. https://doi.org/10.1186/1467-4866-14-1
Riegel M, Tokmachev M, Hoell WH (2008) Kinetics of uranium sorption onto weakly basic anion exchangers. React Funct Polym 68:1072–1080. https://doi.org/10.1016/j.reactfunctpolym.2008.02.009
Kowalczyk M, Hubicki Z, Kołodyńska D (2013) Removal of heavy metal ions in the presence of the biodegradable complexing agent of EDDS from waters. Chem Eng J 221:512–521. https://doi.org/10.1016/j.cej.2013.02.010
Riegel M, Schlitt V (2017) Sorption dynamics of uranium onto anion exchangers. Water 9:268. https://doi.org/10.3390/w9040268
Koodynska D, Hubicki Z (2012) Investigation of sorption and separation of lanthanides on the ion exchangers of various types. In: Kilislioglu A (ed) Ion exchange technologies. InTech
Pehlivan E, Cetin S (2009) Sorption of Cr(VI) ions on two Lewatit-anion exchange resins and their quantitative determination using UV–visible spectrophotometer. J Hazard Mater 163:448–453. https://doi.org/10.1016/j.jhazmat.2008.06.115
Galán B, Calzada M, Ortiz I (2006) Separation and Concentration of Cr(VI) from ground waters by anion exchange using lewatit MP-64: mathematical modelling at acidic pH. Solv Extr Ion Exchange 24:621–637. https://doi.org/10.1080/07366290600762413
Purkayastha D, Mishra U, Biswas S (2014) A comprehensive review on Cd(II) removal from aqueous solution. J Water Process Eng 2:105–128. https://doi.org/10.1016/j.jwpe.2014.05.009
Khawassek Y (2017) Anion exchange of uranium from sulfuric acid solution: adsorption and kinetics characteristics. In: ALTA 2017 URANIUM-REE SESSIONS. https://www.researchgate.net/publication/328062508_ANION_EXCHANGE_OF_URANIUM_FROM_SULFURIC_ACID_SOLUTION_ADSORPTION_AND_KINETICS_CHARACTERISTICS. Accessed 23 Dec 2019
Orabi AH, Rabia K, Elshereafy E, Salem A (2017) Application of commercial adsorbent for rare earth elements—uranium mutual separation and purification. MediterrJChem 6:238. https://doi.org/10.13171/mjc66/01712211014-orabi
Afifi SY, Elashry SM, Abo-Aly MM (2017) Alkaline leaching for recovery of uranium and copper from calcareous shale, um bogma formation, g. allouga, southwestern sinai. Egypt Arab J Nucl Sci Appl 50:213–228
Kołodyńska D, Hubicki Z, Skiba A (2009) Heavy metal ions removal in the presence of 1-hydroxyethane-1,1-diphosphonic acid from aqueous solutions on polystyrene anion exchangers. Ind Eng Chem Res 48:10584–10593. https://doi.org/10.1021/ie901195j
Kadous A, Didi MA, Villemin D (2011) Removal of uranium(VI) from acetate medium using Lewatit TP 260 resin. J Radioanal Nucl Chem 288:553–561. https://doi.org/10.1007/s10967-010-0970-1
Wołowicz A, Hubicki Z (2012) The use of the chelating resin of a new generation Lewatit MonoPlus TP-220 with the bis-picolylamine functional groups in the removal of selected metal ions from acidic solutions. Chem Eng J 197:493–508. https://doi.org/10.1016/j.cej.2012.05.047
Liu Y, Cao X, Le Z et al (2010) Pre-concentration and determination of trace uranium (VI) in environments using ion-imprinted chitosan resin via solid phase extraction. J Braz Chem Soc 21:533–540. https://doi.org/10.1590/S0103-50532010000300020
Praveen R, Metilda P, Daniel S, Rao T (2005) Solid phase extractive preconcentration of uranium(VI) using quinoline-8-ol anchored chloromethylated polymeric resin beads. Talanta 67:960–967. https://doi.org/10.1016/j.talanta.2005.04.019
Kouraim MN, Hagag MS, Ali AH (2019) Sorption of uranium from radioactive wastes by silicate-neutralised polyacrylic. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2019.1641600
Sartore L, Dey K (2019) Preparation and heavy metal ions chelating properties of multifunctional polymer-grafted silica hybrid materials. Adv Mater Sci Eng 2019:1–11. https://doi.org/10.1155/2019/7260851
Massey MS, Lezama-Pacheco JS, Nelson JM et al (2014) Uranium incorporation into amorphous silica. Environ Sci Technol 48:8636–8644. https://doi.org/10.1021/es501064m
Zhang S, Li J, Wen T et al (2013) Magnetic Fe3O4@NiO hierarchical structures: preparation and their excellent As(v) and Cr(vi) removal capabilities. RSC Adv 3:2754. https://doi.org/10.1039/c2ra22495j
Shapiro L, Brannock WW (1962) Rapid analysis of silicate, carbonate, and phosphate rocks. US Geological Survey Bulletin 114A (Revised Edition): https://doi.org/10.3133/b1144A
Kuppusami Govindaraju, Guy Mevelle, Charles Chouard (1976) Automated optical emission spectrochemical bulk analysis of silicate rocks with microwave plasma excitation. Anal Chem 48:1325–1331. https://doi.org/10.1021/ac50003a018
Marczenko Z, Balcerzak M (2000) Principles of Spectrophotometry. In: Analytical spectroscopy library. Elsevier, pp 26–38
Davies W (1964) A rapid and specific titrimetric method for the precise determination of uranium using iron(II) sulphate as reductant. Talanta 11:1203–1211. https://doi.org/10.1016/0039-9140(64)80171-5
Mathew KJ, Bürger S, Vogt S et al (2009) Uranium assay determination using Davies and Gray titration: an overview and implementation of GUM for uncertainty evaluation. J Radioanal Nucl Chem 282:939–944. https://doi.org/10.1007/s10967-009-0186-4
Cheira MF, Atia BM, Kouraim MN (2017) Uranium(VI) recovery from acidic leach liquor by Ambersep 920U SO4 resin: kinetic, equilibrium and thermodynamic studies. J Radiat Res Appl Sci 10:307–319. https://doi.org/10.1016/j.jrras.2017.07.005
Cheira MF, Orabi AS, Atia BM, Hassan SM (2018) Solvent extraction and separation of thorium(iv) from chloride media by a schiff base. J Solution Chem 47:611–633. https://doi.org/10.1007/s10953-018-0740-1
Blanchard G, Maunaye M, Martin G (1984) Removal of heavy metals from waters by means of natural zeolites. Water Res 18:1501–1507. https://doi.org/10.1016/0043-1354(84)90124-6
Weber J, Morris JC, Weber W et al (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div A Soc Civil Eng 89:31–60
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403. https://doi.org/10.1021/ja02242a004
Freundlich H (1907) Adsorption in solution. Zeitschrift für Physikalische Chemie. https://doi.org/10.1515/zpch-1907-5723
Das DP, Das J, Parida K (2003) Physicochemical characterization and adsorption behavior of calcined Zn/Al hydrotalcite-like compound (HTlc) towards removal of fluoride from aqueous solution. J Colloid Interface Sci 261:213–220. https://doi.org/10.1016/S0021-9797(03)00082-1
Liao X, Shi B (2005) Adsorption of Fluoride on Zirconium(IV)-Impregnated Collagen Fiber. Environ Sci Technol 39:4628–4632. https://doi.org/10.1021/es0479944
Chaudhary N, Balomajumder C (2014) Optimization study of adsorption parameters for removal of phenol on aluminum impregnated fly ash using response surface methodology. J Taiwan Inst Chem Eng 45:852–859. https://doi.org/10.1016/j.jtice.2013.08.016
Atia BM, Gado MA, Abd El-Magied MO, Elshehy EA (2019) Highly efficient extraction of uranyl ions from aqueous solutions using multi-chelators functionalized graphene oxide. Sep Sci Technol. https://doi.org/10.1080/01496395.2019.1650769
Kannamba B, Reddy KL, AppaRao BV (2010) Removal of Cu(II) from aqueous solutions using chemically modified chitosan. J Hazard Mater 175:939–948. https://doi.org/10.1016/j.jhazmat.2009.10.098
Dong J, Ozaki Y (1997) FTIR and FT-raman studies of partially miscible poly(methyl methacrylate)/poly(4-vinylphenol) blends in solid states. Macromolecules 30:286–292. https://doi.org/10.1021/ma9607168
Khalifa ME (1998) Selective separation of uranium using alizarin red S (ARS)-modified anion-exchange resin or by flotation of U-ARS chelate. Sep Sci Technol 33:2123–2141. https://doi.org/10.1080/01496399808545719
Sid Kalal H, Panahi HA, Hoveidi H et al (2012) Synthesis and application of Amberlite xad-4 functionalized with alizarin red-s for preconcentration and adsorption of rhodium (III). J Environ Health Sci Engineer 9:7. https://doi.org/10.1186/1735-2746-9-7
Anirudhan TS, Rijith S (2012) Synthesis and characterization of carboxyl terminated poly(methacrylic acid) grafted chitosan/bentonite composite and its application for the recovery of uranium(VI) from aqueous media. J Environ Radioact 106:8–19. https://doi.org/10.1016/j.jenvrad.2011.10.013
Atrees MS, Metwally E, Demerdash M, Salem H (2016) Sorption behavior of Pr and Nd upon chitosan benzoyl thiourea derivatives. J Radiat Res Appl Sci 9:207–216. https://doi.org/10.1016/j.jrras.2015.02.004
Solgy M, Taghizadeh M, Ghoddocynejad D (2015) Adsorption of uranium(VI) from sulphate solutions using Amberlite IRA-402 resin: equilibrium, kinetics and thermodynamics study. Ann Nucl Energy 75:132–138. https://doi.org/10.1016/j.anucene.2014.08.009
Massoud A, Masoud AM, Youssef WM (2019) Sorption characteristics of uranium from sulfate leach liquor by commercial strong base anion exchange resins. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-019-06770-9
Semnani F, Asadi Z, Samadfam M, Sepehrian H (2012) Uranium(VI) sorption behavior onto amberlite CG-400 anion exchange resin: effects of pH, contact time, temperature and presence of phosphate. Ann Nucl Energy 48:21–24. https://doi.org/10.1016/j.anucene.2012.05.010
Ladeira ACQ, Gonçalves CR (2007) Influence of anionic species on uranium separation from acid mine water using strong base resins. J Hazard Mater 148:499–504. https://doi.org/10.1016/j.jhazmat.2007.03.003
Mattigod SV, Golovich EC, Wellman DM, et al (2010) Uranium Adsorption on Ion-Exchange Resins—Batch Testing
Kosari M, Sepehrian H (2016) Uranium Removal from Aqueous Solution Using Ion-Exchange Resin DOWEX® 2x8 in the Presence of Sulfate Anions. Int J Eng 29:1677–1683
Cheira FM, El-Didamony AM, Mahmoud FK, Atia BM (2014) Equilibrium and kinetic characteristics of uranium recovery by the strong base ambersep 920U Cl resin. IOSRJAC 7:32–40. https://doi.org/10.9790/5736-07533240
Balanovsky NV, Koshcheeva AM, Cherednichenko AG (2016) Synthesis and properties of strongly basic acrylate polyfunctional anion-exchange resin for uranium extraction. Moscow Univ Chem Bull 71:336–340. https://doi.org/10.3103/S0027131416050023
Khawassek YM, Eliwa AA, Haggag EA et al (2017) Equilibrium, kinetic and thermodynamics of uranium adsorption by ambersep 400 SO4 resin. Arab J Nucl Sci Appl 50:100–112
Venkatesan KA, Shyamala KV, Antony MP et al (2008) Batch and dynamic extraction of uranium(VI) from nitric acid medium by commercial phosphinic acid resin, Tulsion CH-96. J Radioanal Nucl Chem 275:563–570. https://doi.org/10.1007/s10967-007-6888-6
Acnowledgements
This work was funded by the Nuclear Materials Authority as a part of its research activities. This article was reviewed and approved for publishing by the Nuclear Materials Authority with no obligation on the authors’ part to revise the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmad, A.A. Kinetics of uranium adsorption from sulfate medium by a commercial anion exchanger modified with quinoline and silicate. J Radioanal Nucl Chem 324, 1387–1403 (2020). https://doi.org/10.1007/s10967-020-07169-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07169-7