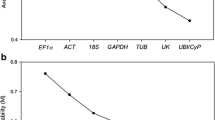
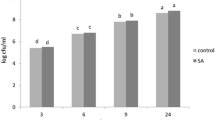
Abstract
Alternaria blight causes significant losses in tomato. High disease severity during fruiting stage restricts the applicability of chemicals. Chemical pesticides can be substituted by resistance inducing agents. In present investigation, two resistance inducing agents namely Salicylic acid and Abscisic acid and a plant growth promoting rhizobacteria Pseudomonas fluorescens strain PBAT-2 (Psf) were used to assess their effect on Alternaria blight disease severity through the activity of defense-related compounds i.e. Peroxidase (POD), Polyphenol oxidase (PPO), Phenyl ammonia lyase (PAL), total phenolic content along with pathogenesis-related gene PR-1 and β-1,3-glucanase (GLU) gene expression in tomato. SA, ABA and Psf reduced disease severity significantly as compared to control. A significant increase in the activity of POD, PPO, PAL and total phenol content was recorded in all the three treatments over control. POD, PPO, PAL levels were significantly high at 24 h and 48 h post Alternaria solani inoculation, whereas total phenolic content increased up to 72 h. The expression of PR-1 and GLU gene was upregulated at 24 h post A. solani inoculation in SA and ABA treated leaves. However, these genes were unexpressed in Psf treated plants which confirms the role of SA and ABA in systemic acquired resistance. These findings suggest that SA, ABA and Psf can be used in early blight management in tomato without affecting the quality of the fruit.





Similar content being viewed by others
Abbreviations
- µg:
-
Micro gram
- µL:
-
Micro litre
- ABA:
-
Abscissic acid
- GLU:
-
β-1, 3-Glucanase
- ISR:
-
Induced systemic resistance
- PAL:
-
Phenyl ammonia lyase
- PDI:
-
Per cent Disease Index
- POD:
-
Peroxidase
- PPO:
-
Polyphenol oxidase
- PR-1:
-
Pathogenesis related
- Psf:
-
Pseudomonas fluorescens
- SA:
-
Salicylic acid
- SAR:
-
Systemic acquired resistance
- U:
-
Unit
References
Abo-Elyousr KAM, Ibrahim YE, Balabe NM (2012) Induction of disease defensive enzymes in response to treatment with acibenzolar-S-methyl (ASM) and Pseudomonas fluorescens Pf2 and Inoculation with Ralstonia solanacearum race 3, biovar 2 (phylotype II). J Phytopathol 160:382–389
Achuo EA, Prinsen E, Hofte M (2006) Influence of drought, salt stress and abscisic acid on the resistance of tomato to Botrytis cinerea and Oidium neolycopersici. Plant Pathol 55:178–186
Adie BAT, Pérez-Pérez J, Pérez-Pérez MM, Godoy M, Sánchez-Serrano JJ, Schmelz EA, Solano R (2007) ABA is an essential signal for plant resistance to pathogens affecting JA biosynthesis and the activation of defenses in Arabidopsis. Plant Cell 19:1665–1681
Aime S, Cordier C, Alabouvette C, Olivain C (2008) Comparative analysis of PR gene expression in tomato inoculated with virulent Fusarium oxysporum f. sp. lycopersici and the biocontrol strain F. oxysporum Fo47. Physiol Mol Plant Pathol 73:9–15
Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, Maclean DJ, Ebert PR, Kazan K (2004) Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 16:3460–3479
Asselbergh B, Achuo AE, Höfte M, Van Gijsegem F (2008) Abscisic acid deficiency leads to rapid activation of tomato defense responses upon infection with Erwinia chrysanthemi. Mol Plant Pathol 9:11–24
Audenaert K, De Meyer GB, Hofte MM (2002) Abscisic acid determines basal susceptibility of tomato to Botrytis cinerea and suppresses salicylic acid-dependent signaling mechanisms. Plant Physiol 128:491–501
Bari R, Jones JDG (2009) Role of plant hormones in plant defence responses. Plant Mol Biol 69:473–488
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:246–254
Chinnusamy V, Gong ZZ, Zhu JK (2008) Abscisic acid-mediated epigenetic processes in plant development and stress responses. J Integr Plant Biol 50:1187–1195
Datar VV, Mayee CD (1981) Assessment of losses in tomato yield due to early blight. Indian phytopath 34:191–195
de Torres-Zabala M, Truman W, Bennett MH (2007) Pseudomonas syringae pv. tomato hijacks the Arabidopsis abscisic acid signaling pathway to cause disease. EMBOJ 26:1434–1443
Derksen H, Badawi M, Henriquez MA, Yao Z, El-Bebany AF, Daayf F (2013) Differential expression of potato defence genes associated with the salicylic acid defence signalling pathway in response to weakly and highly aggressive isolates of Verticillium dahliae. J Phytopathol 161:142–153
Durrant WE, Dong X (2004) Systemic acquired resistance. Annu Rev Phytopathol 42:185–209
Edwards R and Kessmann H (1992) Isoflavonoid phytoalexins and their biosynthetic enzymes. In: Molecular plant pathology: a practical approach, pp 45–62
El-Mohamedy RSR, Jabnoun-Khiareddine H, Daami-Remadi M (2014a) Control of root rot diseases of tomato plants caused by Fusarium solani, Rhizoctonia solani and Sclerotium rolfsii using different chemical plant resistance inducers. Tunis J Plant Protect 9:45–55
Flors V, Ton J, Jakab G, Mauch-Mani B (2005) Abscisic acid and callose: team players in defence against pathogens. J Phytopathol 153:377–383
Friedrich L, Lawton K, Dincher S, Winter A, Staub T, Uknes S, Kessman H, Ryals J (1996) Benzothiadiazole induces systemic acquired resistance in tobacco. Plant J 10:61–70
Funnell DL, Lawrence CB, Pedersen JF, Schardl CL (2004) Expression of the tobacco β-1,3-glucanase gene, PR-2d, following induction of SAR with Peronospora tabacina. Physiol Mol Plant Pathol 65:285–296
Grogan RG, Kimble KA, Misaghi J (1975) A stem canker disease of tomato caused by Alternaria alternata f. sp. lycopersici. Phytopathol 65:880–886
Herman MAB, Davidson JK, Smart CD (2008) Induction of plant defense gene expression by plant activators and Pseudomonas syringae pv tomato in greenhouse-grown tomatoes. Phytopathology 98:1226–1232
Jendoubi W, Harbaoui K, Hamada W (2015) Salicylic acid-induced resistance against Fusarium oxysporum f.sp radicislycopercisi in hydroponic grown tomato plants. J New Sci Agric Biotechnol 21(5):985–995
Jones JB, Jones JP, Stall RE, Zitter TA (1991) Infectious diseases: diseases caused by fungi. In: Compendium of tomato diseases. The American Phytopathological Society, St. Paul, pp 9–25
Kumar A, Gond SK, Mishra A, Sharma VK, Verma SK, Singh DK, Kumar J, Kharwar RN (2015) Salicylic acid and its role in systemic resistance induced by Pseudomonas fluorescens to early blight disease of tomato. Vegetos 28(3):12–19
Lanna-Filho R, Souza RM, Magalhães MM, Villela L, Zanotto E, Pedro MR, Resende MLV (2013) Induced defense responses in tomato against bacterial spot by proteins synthesized by endophytic bacteria. Trop Plant Pathol 38(4):295–302
Lavrova VV, Zinovieva SV, UdalovaZhV MEM (2017) Expression of PR genes in tomato tissues infected by nematode Meloidogyne incognita (Kofoid et White, 1919) Chitwood, 1949. Dokl Biochem Biophys 476:306–309
Li L, Zou Y (2017) Induction of disease resistance by salicylic acid and calcium ion against Botrytis cinerea in tomato (Lycopersicon esculentum). Emir J Food Agric 29(1):78–82
Li XP, Xu QQ, Liao WB, Ma ZJ, Xu XT, Wang M, Ren PJ, Niu LJ, Jin X, Zhu YC (2016) Hydrogen peroxide is involved in abscisic acid-induced adventitious rooting in cucumber (Cucumis sativus L.) under drought stress. J Plant Biol 59(5):536–548
Liu J (2012) Induced resistance of tomato against gray mold (Botrytis cinerea) by Salicylic acid (SA). Plant Diseases Pest 3(2):60–63
Lovelock DA, Donald CE, Conlan XA, Cahill DM (2013) Salicylic acid suppression of clubroot in broccoli (Brassicae oleracea var. italica) caused by the obligate biotroph Plasmodiophora brassicae. Australas Plant Pathol 42:141–153
Lyon G (2007) Agents that can elicit induced resistance. In: Walters D, Newton A, Lyon G (eds) Induced resistance for plant disease control: a sustainable approach to crop protection. Blackwell Publishing, Oxford, pp 9–29
Mandal S, Kar I, Mukherjee AK, Acharya P (2013) Elicitor-induced defense responses in Solanum lycopersicum against Ralstonia solanacearum. Sci World J 1–9
Mandal S, Mallick N, Mitra A (2009) Salicylic acid-induced resistance to Fusarium oxysporum f.sp. lycopersici in tomato. Plant Physiol Biochem 47:642–649
Mauch-Mani B, Mauch F (2005) The role of abscisic acid in plant-pathogen interactions. Curr Opin Plant Biol 8:409–414
Mayer AM, Harel E, Shaul RB (1965) Assay of catechol oxidase, a critical comparison of methods. Phytochemistry 5:783–789
Melotto M, Underwood W, Koczan J, Nomura K, He SY (2006) Plant stomata function in innate immunity against bacterial invasion. Cell 126:969–980
Mohr PG, Cahill DM (2007) Suppression by ABA of salicylic acid and lignin accumulation and the expression of multiple genes, in Arabidopsis infected with Pseudomonas syringae pv. tomato. Funct Integr Genomics 7:181–191
Molinari S, Fanelli E, Leonetti P (2013) Expression of tomato salicylic acid (SA)-responsive pathogenesis-related genes in Mi-1- mediated and SA-induced resistance to root-knot nematodes. Mol Plant Pathol 15(3):255–264
Mostafanezhad H, Sahebani N, Zarghani SN (2014) Induction of resistance in tomato against root-knot nematode Meloidogyne javanica with salicylic acid. J Crop Prot 3(4):499–508
Murthy NK, Uzma F, Chitrashree SC (2014) Induction of systemic resistance in tomato against Ralstonia solanacearum by Pseudomonas fluorescens. Am J Plant Sci 5:1799–1811
Obradovic A, Jones JB, Momol MT, Olson SM, Jackson LE, Balogh B, Guven K, Iriarte FB (2005) Integration of biological control agents and systemic acquired resistance inducers against bacterial spot on tomato. Plant Dis 89:712–716
Ojha S, Chatterjee NC (2012) Induction of resistance in tomato plants against Fusarium oxysporum f.sp. lycopersici mediated through salicylic acid and Trichoderma harzianum. J Plant Protect Res 52(2):220–225
Ong S, Cruz FCS (2016) Effect of exogenous application of salicylic acid on the severity of tomato leaf curl disease. J ISSAAS 22(1):137–145
Pandey KK, Pandey PK (2003) Survey and Surveillance of vegetable growing area for prevalence of major diseases in this region. Veg Sci 30:128–134
Pradhanang PM, Ji P, Momol MT, Olson SM, Mayfield JL, Jones JB (2005a) Application of acibenzolar-S-methyl enhances host resistance in tomato against Ralstonia solanacearum. Plant Dis 89:989–993
Prakash N, Vishunavat K (2017) Enhancing sporulation and determination of virulence of Alternaria solani isolates infecting tomato. Indian Phytopath 70(4):471–477
Prasad P, Kumar J (2012) RNA isolation protocol for recovery of high quality functional RNA from fungi and plants. Curr Sci 102(9):1257–1260
Ramamoorthy V, Raguchander T, Samiyappan R (2002) Induction of defense-related proteins in tomato roots treated with Pseudomonas fluorescensPf1 and Fusarium oxysporum f. sp.lycopersici. Plant Soil 239:55–68
Sahi HPS, Shyam KR (1993) Occurrence of Alternaria leaf spots on tomato in Himanchal Pradesh—a new record. Plant Dis Res 8:140–141
Sayari M, Babaeizad V, Ghanbari MAT, Rahimian H (2014) Expression of the pathogenesis related proteins, NH-1, PAL, and lipoxygenase in the Iranian Tarom and Khazar rice cultivars, in reaction to Rhizoctonia solani—the causal agent of rice sheath blight. J Plant Protect Res 54(1):36–43
Silva HSA, Romeiro RS, Macagnan D, Halfeld-Vieira B, Pereira MCB, Mounteer A (2004) Rhizobacterial induction of systemic resistance in tomato plants: non-specific protection and increase in enzyme activities. Biol Control 29:288–295
Silva RF, Pascholati SF, Bedendo IP (2013) Induced resistance in tomato plants to Clavibacter michiganensis subsp. michiganensis by Lentinula edodes and Agaricus subrufescens (syn. Agaricus brasiliensis). J Plant Pathol 95(2):285–297
Singh PC, Kumar R, Singh M, Rai A, Singh MC, Rai M (2011) Identification of resistant sources against early blight disease of tomato. Indian J Hort 68(4):516–521
Song W, Ma X, Tan H, Zhou J (2011) Abscisic acid enhances resistance to Alternaria solani in tomato seedlings. Plant Physiol Biochem 49:693–700
Spoel SH, Dong X (2012) How do plants achieve immunity? defence without specialized immune cells. Nat Rev Immunol 12:89–100
Suresh A, Pallavi P, Srinivas P, Kumar VP, Chandra SJ, Reddy SR (2010) Plant growth promoting activities of fluorescent pseudomonads associated with some crop plants. Afr J Microbiol Res 4:1491–1494
Swain T, Hillis WE (1959) The phenolic constituents of Purmus domestica. The quantitative analysis of phenolic constituents. J Food Sci Agric 10:63–68
Tatiana Z, Yamashita K, Matsumoto H (1999) Iron deficiency induced changes in ascorbate content and enzyme activities related to ascorbate metabolism in cucumber roots. Plant Cell Physiol 40:273–280
Thaler JS, Bostock RM (2004) Interactions between abscisicacid mediated responses and plant resistance to pathogens and insects. Ecology 85:48–58
Tian SP, Chan ZL (2004) Potential of induced resistance in post-harvest diseases control of fruits and vegetables. Acta Phytopathol Sinica 34:385–394
Ton J, Flors V, Mauch-Mani B (2009) The multifaceted role of ABA in disease resistance. Trends Plant Sci 14:310–317
van Loon LC, Bakker PAHM, Pieterse CMJ (1998) Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol 36:453–483
van Loon LC (2007) Plant responses to plant growth-promoting rhizobacteria. In: New perspectives and approaches in plant growth-promoting Rhizobacteria research. Springer, Dordrecht, pp 243–254
van Wees SCM, Luijendijk M, Smoorenburg I, Van Loon LC, Pieterse CMJ (1999) Rhizobacteria-mediated induced systemic resistance (ISR) in Arabidopsis is not associated with a direct effect on expression of known defense-related genes but stimulates the expression of the jasmonate-inducible gene Atvsp upon challenge. Plant Mol Biol 41:537–549
Vlot A, Dempsey DA, Klessig DF (2009) Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47:177–206
Vloutoglou I, Kalogerakis SN (2000) Effects of inoculum concentration, wetness duration and plant age on development of early blight (Alternaria solani) and on shedding of leaves in tomato plants. Plant Path 49:339–345
Walters DR, Fountaine JM (2009) Practical application of induced resistance to plant diseases: an appraisal of effectiveness under field conditions. J Agric Sci 147:523–535
Wang Y, Tao X, Tang XM, Xiao L, Sun J, Yan XF, Li D, Deng HY, Ma XR (2013) Comparative transcriptome analysis of tomato (Solanum lycopersicum) in response to exogenous abscisic acid. BMC Genomics 14:841
Weller DM, Mavrodi DV, van Pelt JA, Pieterse CMJ, vanLoon LC, Bakker PAHM (2012) Induced systemic resistance in Arabidopsis thaliana against Pseudomonas syringae pv. tomato by 2,4-diacetylphloroglucinol-producing Pseudomonas fluorescens. Phytopathology 102:403–412
Wilson CL, Wisniewski ME (1989) Biological control of postharvest diseases of fruits and vegetables: An emerging technology. Annu Rev Phytopathol 27:425–441
Yan ZN, Reddy MS, Ryu CM, McInroy JA, Wilson M, Kloepper JW (2002) Induced systemic protection against tomato late blight elicited by plant growth-promoting rhizobacteria. Phytopathology 92(12):1329–1333
Zhang J, Du X, Wang Q, Chen X, Lv D, Xu K, Qu S, Zhang Z (2010) Expression of pathogenesis related genes in response to salicylic acid, methyl jasmonate and 1-minocyclopropane-1-carboxylic acid in Malus hupehensis (Pamp.) Rehd. BMC Res Notes 3:208
Zhao LM, Zheng ZX, Zhao X, Shi J, Bi JJ, Pei W, Feng Q (2014) Optimization of reference genes for normalization of the quantitative polymerase chain reaction in tissue samples of gastric cancer. Asian Pac J Cancer Prev 15:5815–5818
Acknowledgement
This research programme was funded by Seed Pathology Laboratory, Department of Plant Pathology, GBPUA&T, Pantnagar. We extend our gratitude to the In-charge, Stress Laboratory, Department of Molecular Biology and Genetic Engineering, GBPUA&T, Pantnagar for providing all laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Prakash, N., Vishunavat, K., Khan, G.T. et al. SA, ABA and Pseudomonas fluorescens elicit defense responses in tomato against Alternaria blight. J. Plant Biochem. Biotechnol. 30, 13–25 (2021). https://doi.org/10.1007/s13562-020-00564-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13562-020-00564-x