Abstract
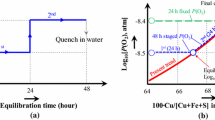
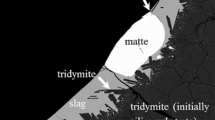
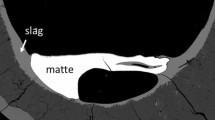
The effect of temperature, CaO, MgO and Al2O3 on important technological copper smelting parameters, such as the chemically dissolved copper in slag and the composition of the liquid phase in equilibrium with tridymite, are experimentally characterised as a function of copper concentration in matte. Two series of experiments for the gas/slag/matte/tridymite equilibria in the Cu-Fe-O-S-Si system at p(SO2) = 0.25 atm have been carried out. The effect of CaO at 1573 K (1300 °C), and the combined effect of Al2O3 + CaO + MgO at 1473 K (1200 °C) and 1573 K (1300 °C) have been measured in the first and second series of experiments respectively. The experimental methodology involves high temperature equilibration of samples on a substrate made from the primary phase under controlled gas atmosphere (CO/CO2/SO2/Ar), followed by rapid quenching of the equilibrium condensed phases and direct measurement of the phase compositions using the Electron Probe x-ray Microanalysis. The resulting data are used in the optimization of the thermodynamic database for the copper-containing systems.







Similar content being viewed by others
References
A. Yazawa and M. Kameda, Fundamental Studies on Copper Smelting. IV. Solubility of FeO in Copper Matte from SiO2-saturated FeO-SiO2 Slag, Technol. Rep. Tohoku Univ., 1955, 19(2), p 251-261
A. Yazawa and M. Kameda, Fundamental Studies on Copper Smelting. III. Partial Liquidus Diagram for Cu2S-FeS-FeO System, Technol. Rep. Tohoku Univ., 1955, 19(2), p 239-250
N. Korakas, Etude thermodynamic de l’équilibre entre scories ferro-siliceuses et mattes de cuivre. Application aux problèmes posés par la formation de magnetite lors du traitement des minerais sulfurés de cuivre, Univirsité de Liège, Liège, 1964
M. Kameda and A. Yazawa, The Oxygen Content of Copper Mattes. In Physical Chemistry of Process Metallurgy, part 2, 1961. TMS Conference on Proceedings Interscience, N.Y
U. Kuxmann and F.Y. Bor, Studies on the Solubility of Oxygen in Copper Mattes under Ferric Oxide Slags Saturated with Silica, Erzmetall, 1965, 18, p 441-450
F.Y. Bor and P. Tarassoff, Solubility of Oxygen in Copper Mattes, Can. Metall. Q., 1971, 10(4), p 267-271
A. Geveci and T. Rosenqvist, Equilibrium Relations Between Liquid Copper, Iron-Copper Matte, and Iron Silicate Slag at 1250o, Trans. Inst. Min. Metall., 1973, 82, p C193-C201
M. Nagamori, Metal Loss to Slag: Part I. Sulfidic and Oxidic Dissolution of Copper in Fayalite Slag from Low Grade Matte, Metall. Trans. B, 1974, 5(3), p 531-538
H. Jalkanen, Copper and Sulfur Solubilities in Silica-Saturated Iron Silicate Slags from Copper Mattes, Scand. J. Metall., 1981, 10(4), p 177-184
A. Yazawa, S. Nakazawa, and Y. Takeda, Distribution Behavior of Various Elements in Copper Smelting Systems. In Advances in Sulfide Smelting. Proceedings of the 1983 International Sulfide Smelting Symposium and the 1983 Extractive and Process Metallurgy Meeting of the Metallurgical Society of AIME
R. Shimpo et al., A Study on the Equilibrium between Copper Matte and Slag, Can. Metall. Q., 1986, 25(2), p 113-121
F.J. Tavera and E. Bedolla, Distribution of Copper, Sulfur, Oxygen and Minor Elements Between Silica-Saturated Slag, Matte and Copper - Experimental Measurements, Int. J. Miner. Process., 1990, 29(3–4), p 289-309
H. Li and W.J. Rankin, Thermodynamics and Phase Relations of the Fe-O-S-SiO2(sat) System at 1200 °C and the Effect of Copper, Metall. Trans. B, 1994, 25B(1), p 79-89
Y. Takeda, Copper solubility in matte smelting slag. In Proceedings of the International Conference on Molten Slags, Fluxes Salts ‘97, 5th. 1997. Iron and Steel Society Warrendale, PA
J.M. Font, M. Hino, and K. Itagaki, Phase Equilibrium and Minor Elements Distribution between Iron-Silicate Base Slag and Nickel-Copper-Iron Matte at 1573 K under High Partial Pressures of SO2, Mater. Trans., 1999, 40(1), p 20-26
Y. Takeda, Oxygen potential measurement of iron silicate slag-copper-matte system. In Proceedings of the International Conference on Molten Slags, Fluxes Salts ‘97, 5th 1997. Iron and Steel Society Warrendale, PA
A. Fallah-Mehrjardi, P.C. Hayes, and E. Jak, Experimental Investigation of Gas/Slag/Matte/Tridymite Equilibria in the Cu-Fe-O-S-Si System in Controlled Gas Atmospheres: Experimental Results at T = 1473 K [1200°C] and P(SO2) = 0.1 atm. Int. J. Mater. Res., 2018 (submitted)
A. Fallah-Mehrjardi et al., Experimental Investigation of Gas/Slag/Matte/Tridymite Equilibria in the Cu-Fe-O-S-Si system in Controlled Gas Atmospheres: Development of Technique, Metall. Mater. Trans. B, 2017, 48(6), p 3002-3016
A. Fallah-Mehrjardi et al., Experimental Investigation of Gas/Slag/Matte/Tridymite Equilibria in the Cu-Fe-O-S-Si System in Controlled Gas Atmospheres: Experimental Results at T = 1473 K [1200°C] and P(SO2) = 0.25 atm, Metall. Mater. Trans. B, 2017, 48(6), p 3017-3026
Jak, E., et al., Integrated experimental and modelling research for non-ferrous smelting and recycling systems. In Proceedings of the 10th International Conference on Molten Slags, Fluxes and Salts (Molten16), R.G. Reddy, et al., Editors. 2016: Seattle, Washington, USA. p. 947–959.
D. Shishin, E. Jak, and S.A. Decterov, Critical Assessment and Thermodynamic Modeling of the Fe-O-S System, J. Phase Equilib. Diffus., 2015, 36(3), p 224-240
D. Shishin, E. Jak, and S.A. Decterov, Thermodynamic Assessment and Database for the Cu–Fe–O–S System, CALPHAD, 2015, 50, p 144-160
T. Hidayat et al., Critical Assessment and Thermodynamic Modeling of the Cu-Fe-O-Si System, CALPHAD, 2017, 58, p 101-114
D. Shishin, S.A. Decterov, and E. Jak, Thermodynamic Assessment of Slag-Matte-Metal Equilibria in the Cu–Fe–O–S–Si System, J. Phase Equilib. Differ., 2018, 39(5), p 456-475
D. Shishin et al., Integrated Experimental and Thermodynamic Modelling Study of the Effects of Al2O3, CaO and MgO on Slag–Matte Equilibria in the Cu-Fe-O-S-Si-(Al, Ca, Mg) System, J. Phase Equilib. Differ., 2018, 10, p 1-17
T. Hidayat et al., Experimental Study and Thermodynamic Re-Evaluation of the FeO-Fe2O3-SiO2 System, J. Phase Equilib. Diffus., 2017, 38(4), p 477-492
D. Shishin, P.C. Hayes, and E. Jak. Development and applications of thermodynamic database in copper smelting. In Copper’19 conference. 2019. Vancouver, Canada.
A. Fallah-Mehrjardi, P.C. Hayes, and E. Jak, The Effect of CaO on Gas/Slag/Matte/Tridymite Equilibria in Fayalite-Based Copper Smelting Slags at 1473 K (1200°C) and P(SO2) = 0.25 Atm, Metall. Mater. Trans. B, 2017, 49(2), p 602-609
C.W. Bale et al., FactSage Thermochemical Software and Databases, 2010–2016, CALPHAD, 2016, 54, p 35-53
M. Allibert, et al., Slag Atlas, 2nd Edition. 1995: Verlag Stahleisen GmbH, d-Dusseldorf, on behalf of the European Communities, Germany.
F. Tsukihashi and H. Kimura. The effect of MgO and Al2O3 additions on the liquidus of the CaO-SiO2-FeOx system at various partial pressure of oxygen. In Metallurgical and Materials Processing: Principles and Technologies, Yazawa International Symposium, San Diego, CA, United States, March 2–6. 2003. San Diego, CA, United States: Minerals, Metals & Materials Society, Warrendale, PA
Acknowledgments
The authors would like to thank Australian Research Council Linkage program LP 140100480, Altonorte Glencore, Atlantic Copper, Aurubis, Olympic Dam Operation BHP Billiton, Kazzinc Glencore, PASAR Glencore, Outotec Oy (Espoo), Anglo American Platinum, Umicore, and Kennecott Rio Tinto for the financial and technical support for this study. The authors would like to thank the Centre for Microscopy and Microanalysis, University of Queensland for technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ata Fallah-Mehrjardi, Taufiq Hidayat: Formerly with PYROSEARCH.
Rights and permissions
About this article
Cite this article
Sineva, S., Fallah-Mehrjardi, A., Hidayat, T. et al. Experimental Investigation of Gas/Slag/Matte/Tridymite Equilibria in the Cu-Fe-O-S-Si-Al-Ca-Mg System in Controlled Gas Atmosphere: Experimental Results at 1473 K (1200 °C), 1573 K (1300 °C) and p(SO2) = 0.25 atm. J. Phase Equilib. Diffus. 41, 243–256 (2020). https://doi.org/10.1007/s11669-020-00810-8
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-020-00810-8