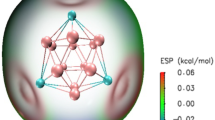
Abstract
The adsorption of carbon dioxide and carbon monoxide molecules on the magnetic cluster C4H8Ti4 was studied within the framework of Density Functional Theory. We have showed the adsorption of eight molecules, each of CO2 and CO on a single cluster-C4H8Ti4. We have discussed binding energy, charge transfer mechanism, change in dipole moment, and HOMO-LUMO analysis.




Similar content being viewed by others
References
Petrova GN, Efimov ON, Strelets VV (1983) Bull Acad Sci USSRCH + 32:1843–1847
Figueroa JD, Fout T, Plasynski S, MeIlvried H, Srivastava RD (2008) Int J Green House Gas Control 2:9–20
Anderson TR, Hawkins E, Jones PD (2016) Endeavour 40:178–187
Tour JM, Kittrell C, Colvin VL (2010) Nat Mater 9:871–874
Song C (2006) Catal Today 115:2–32
Vorosmarty C, Schloss A (1993) Nature 363:359378
Cheng D, Negreiros FR, Apra E, Fortunelli A (2013) ChemSusChem 6:944
Adger WN, Barnett J, Brown K, Marshall N, O Brein K (2013) Nat Clim Chang 3:112–117
Doney SC, Fabrey VJ, Feely RA, Kleypas JA (2009) Annu Rev Mar Sci 1:169–192
World Health Organization (1999) Monitoring ambient air quality for health impact assessment. WHO Regional Publications, European Series. p. 92
Jiang Y, Sun L, Du J, Liu Y, Shi H, Liang Z, Li J (2017) Crystal Growth and Design. 17(4):2090–2096
Yoon YI, Kim YE, Nam SC, Park SY, Chun S, Lee IS, Kim HS (2017) Energy Procedia 114:6240–6245
Cormos AM, Cormos CC (2017) Chem Eng Res Des 123:230–239
Bjerge L-M, Brevik P (2014) Energy Procedia 63:6455–6463
Toda Y, Hirayama H, Kuganathan N, Torrisi A, Sushko PV, Hosono H (2013) Nat Commun 4:2378. https://doi.org/10.1038/ncomms3378
Fukuhara C, Hayakawa K, Suzuki Y, Kawasaki W, Watanabe R (2016) Appl Catal A Gen 532:12–18
Nomura K, Ujihira Y, Hayakawa T, Takehira K (1996) Appl Catal A Gen 137:25–36
Ding J, Bu Y, Ou M, Yu Y, Zhong Q, Fan M (2017) Appl Catal B Environ 202:314–325
Chen DM, Zhang XJ (2019) J Sol St Chm 278:120906
Phan HT, Pham-Ho MP, Nguyen MT (2019) Chem Phys Lett 728:186–194
Grice KA (2017) Coord Chem Rev 336:78–95
Yang Y, Guo Z, Chen XH, Lin J (2019) J Sol St Chm 276:190–193
Suzuki TM, Takayama T, Sato S, Iwase A, Kudo A, Morikawa T (2018) Appl Catal B Environ 224:572–578
Zhou Si, Yang X, Shen Y, Bruce King R, Zhao J (2019) J Alloys and Comounds 80625:698–704
Zhang QY, Zhao QF, Liang XM, Wang XL, Ma FX, Suo BB, Zou WL, Han HX, Song Q, Wu Q, Li YW, Zhu HY (2018) Int J Hydrog Energy 43(2124):9935–9942
Durgun E, Ciraci S, Zhou W, Yildirim T (2006) Phys Rev Lett 97:226102
Shevlin SA, Guo ZX (2009) Chem Soc Rev 38:211–225
I. S. U. GAMESS version 02 AUG 2018
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chahal, A., Abbas, H. Capacity of C4H8Ti4 cluster for adsorption of CO2 and CO: a computational study. J Mol Model 26, 126 (2020). https://doi.org/10.1007/s00894-020-04403-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04403-7