Abstract
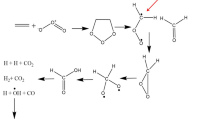
We carried out quantum chemical calculations to analyze the effects of fluorination on the activation energy (Ea) of sulfonic group deprotonation by water molecules. The model molecule was 2,3,4,5,6-pentafluorobenzenesulfonic acid (5FBSA), which was obtained by substituting all aromatic hydrogen atoms of benzenesulfonic acid (BSA) for fluorine atoms. The target hydration level was three. Our analysis indicated that the Ea of deprotonation in 5FBSA was lower than that of BSA, suggesting that the cation of 5FBSA was stabilized. Previous studies have reported that fluorinated molecules have a lower Ea to deprotonation and a stabilized deprotonated state even at a hydration level of three. This effect is attributed to the strong electron withdrawing ability of fluorine. However, compared with non-aromatic molecules, the Ea of deprotonation of aromatic molecules is slightly higher, and the overall energy change (ΔE) is lower, even if the molecule is fluorinated.





Similar content being viewed by others
References
Rikukawa M, Sanui K (2000) Proton-conducting polymer electrolyte membranes based on hydrocarbon polymers. Prog. Polym. Sci. 25:1463–1502 https://doi.org/10.1016/S0079-6700(00)00032-0
Takeoka Y, Umezawa K, Oshima T et al (2014) Synthesis and properties of hydrophilic–hydrophobic diblock copolymer ionomers based on poly(p-phenylene)s. Polym. Chem. 5:4132–4140 https://doi.org/10.1039/C4PY00082J
Umezawa K, Oshima T, Yoshizawa-Fujita M et al (2012) Synthesis of hydrophilic--hydrophobic block copolymer ionomers based on polyphenylenes. ACS Macro Lett. 1:969–972
Souzy R, Ameduri B (2005) Functional fluoropolymers for fuel cell membranes. Prog. Polym. Sci. 30:644–687. https://doi.org/10.1016/j.progpolymsci.2005.03.004
Lindström RW, Oyarce A, Aguinaga LG et al (2013) Performance of phosphonated hydrocarbon ionomer in the fuel cell cathode catalyst layer. J. Electrochem. Soc. 160:F269–F277
Kreuer KD (2001) On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells. J Memb Sci 185:29–39 https://doi.org/10.1016/S0376-7388(00)00632-3
Ghassemzadeh L, Kreuer K-D, Maier J, Müller K (2010) Chemical degradation of Nafion membranes under mimic fuel cell conditions as investigated by solid-state NMR spectroscopy. J. Phys. Chem. C 114:14635–14645
Reiter GF, Kolesnikov AI, Paddison SJ et al (2012) Evidence for an anomalous quantum state of protons in nanoconfined water. Phys. Rev. B 85:45403 https://doi.org/10.1103/PhysRevB.85.045403
Agmon N (1996) Hydrogen bonds, water rotation and proton mobility. J. Chim. Phys. 93:1714–1736
Bae B, Miyatake K, Watanabe M (2009) Effect of the hydrophobic component on the properties of sulfonated poly (arylene ether sulfone) s. Macromolecules 42:1873–1880 https://doi.org/10.1021/ma8026518
Kopitzke RW, Linkous CA, Anderson HR, Nelson GL (2000) Conductivity and water uptake of aromatic-based proton exchange membrane electrolytes. J. Electrochem. Soc. 147:1677–1681
Ghassemi H, McGrath JE, Zawodzinski Jr TA (2006) Multiblock sulfonated--fluorinated poly (arylene ether) s for a proton exchange membrane fuel cell. Polymer (Guildf) 47:4132–4139
Goto K, Rozhanskii I, Yamakawa Y et al (2009) Development of aromatic polymer electrolyte membrane with high conductivity and durability for fuel cell. Polym. J. 41:95 https://doi.org/10.1295/polymj. PJ2008220
Erdogan T, Unveren EE, Inan TY, Birkan B (2009) Well-defined block copolymer ionomers and their blend membranes for proton exchange membrane fuel cell. J Memb Sci 344:172–181 https://doi.org/10.1016/j.memsci.2009.07.048
Essafi W, Gebel G, Mercier R (2004) Sulfonated polyimide ionomers: a structural study. Macromolecules 37:1431–1440 https://doi.org/10.1021/ma034965p
Han SY, Park J, Kim D (2013) Proton-conducting electrolyte membranes based on organosiloxane network/sulfonated poly (ether ether ketone) interpenetrating polymer networks embedding sulfonated mesoporous benzene–silica. J. Power Sources 243:850–858 https://doi.org/10.1016/j.jpowsour.2013.06.072
Lu J, Tang H, Xu C, Jiang SP (2012) Nafion membranes with ordered mesoporous structure and high water retention properties for fuel cell applications. J. Mater. Chem. 22:5810–5819 https://doi.org/10.1039/C2JM14838B
Pulido Ayazo JC, Suleiman D (2012) Supercritical fluid processing of Nafion® membranes: methanol permeability and proton conductivity. J. Appl. Polym. Sci. 124:145–154 https://doi.org/10.1002/app.35098
Zeng J, Zhou Y, Li L, Jiang SP (2011) Phosphotungstic acid functionalized silica nanocomposites with tunable bicontinuous mesoporous structure and superior proton conductivity and stability for fuel cells. Phys. Chem. Chem. Phys. 13:10249–10257 https://doi.org/10.1039/c1cp20076c
Sel O, Azais T, Maréchal M et al (2011) Sulfonic and phosphonic acid and bifunctional organic–inorganic hybrid membranes and their proton conduction properties. Chem. Asian J. 6:2992–3000 https://doi.org/10.1016/j.progpolymsci.2011.06.001
Sakai H, Tokumasu T (2013) Reaction analysis for deprotonation of the sulfonic group of perfluorosulfonic acid molecules at low hydration levels. J. Phys. Chem. A 118:275–282 https://doi.org/10.1021/jp409781s
Sakai H, Tokumasu T (2015) Quantum chemical analysis of the deprotonation of sulfonic acid in a hydrocarbon membrane model at low hydration levels. Solid State Ionics 274:94–99 https://doi.org/10.1016/j.ssi.2015.03.005
Akinori F, Hironori S, Takashi T (2017) Theoretical study of high performance hydrocarbon-based ion-exchange membranes. Comput Theor Chem 1121:44–48 https://doi.org/10.1016/j.comptc.2017.10.008
Frisch MJ, Trucks GW, Schlegel HB, et al Gaussian∼09 {R} evision {E}.01
Momma K, Izumi F (2008) VESTA: a three-dimensional visualization system for electronic and structural analysis. J. Appl. Crystallogr. 41:653–658
Raghavachari K (2000) Perspective on “Density functional thermochemistry. III. The role of exact exchange.” Theor. Chem. Accounts 103:361–363
Bachrach SM (2014) Computational organic chemistry. John Wiley & Sons
Boys SF, de Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 19:553–566
Zawodzinski TA, Derouin C, Radzinski S et al (1993) Water uptake by and transport through Nafion®117 membranes. J. Electrochem. Soc. 140:1041–1047
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 88:899–926
Roy Dennington, Todd A. Keith and JMM (2016) Gauss View, Version6
Wang C, Clark JK, Kumar M, Paddison SJ (2011) An ab initio study of the primary hydration and proton transfer of CF 3SO3H and CF3O(CF2) 2SO3H: effects of the hybrid functional and inclusion of diffuse functions. Solid State Ionics 199–200:6–13 https://doi.org/10.1016/j.ssi.2011.07.002
Acknowledgments
This research is financially supported by the New Energy and Industrial Technology Development Organization (NEDO). All calculations were carried out by the computer of the Advanced Fluid Information Research Center, Tohoku University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fukushima, A., Sakai, H. & Tokumasu, T. Ab initio studies of the effect of the fluorination on deprotonation reaction of the benzene sulfonic acid. J Mol Model 26, 127 (2020). https://doi.org/10.1007/s00894-020-04402-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04402-8