Abstract
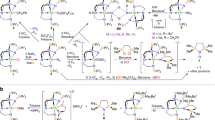
Actinide sulfides are especially significant in actinide chemistry because of their potentials that are used as nuclear fuel and the wide variety of their stoichiometries and physical properties. It is essential for studying the synthesis mechanism of actinide sulfides. In this study, the reactions of thorium cation Th2+ with the facile sulfur-atom donor OCS to produce thorium sulfides have been systematically explored by using density functional. The detailed insights of the primary reaction and secondary reaction paths are reported. We investigated that the multiple bonding characters and complexes involved in reaction exhibit significant covalent character. The reaction rate indicated that the tunneling effect is small compared with the effect of temperature on the rate. This study addresses some of the current limitation in understanding the detailed reaction information of Th2++OCS.





Similar content being viewed by others
References
Arney DSJ, Schnabel RC, And BCS, Bums CJ (1996). J Am Chem Soc 118(28):6780–6781. https://doi.org/10.1021/ja960221y
Wang X, Andrews L, Thanthiriwatte KS, Dixon DA (2013). Inorg Chem 52(18):10275–10285. https://doi.org/10.1021/ic400560k
Li P, Niu W, Gao T (2014). RSC Adv 4:29806–29817. https://doi.org/10.1039/c4ra03525a
Duan M, Li P, Zhao H, Xie F, Ma J (2019). Inorg Chem 58:3425–3434. https://doi.org/10.1021/acs.inorgchem.8b03538
Fortier S, Hayton TW (2010). Coord Chem Rev 254(3–4):197–214. https://doi.org/10.1016/j.ccr.2009.06.003
Hayton TW (2013). Chem Commun 49:2956–2973. https://doi.org/10.1039/C3CC39053E
Wang B, Xia C, Fang H, Chen W, Zhang Y, Huang X (2018). Phys Chem Chem Phys 20:21184–21193. https://doi.org/10.1039/C8CP03071E
Zhao H, Li P, Duan M, Xie F, Ma J (2019). RSC Adv 9:17119–17128. https://doi.org/10.1039/C9RA02098E
Cla’udia CLP, Marsden CJ, Marçalo J, John KG (2011). Phys Chem Chem Phys 13:12940–12958. https://doi.org/10.1039/c1cp20996e
Andrews L, Wang X, Liang B, Ruipérez F, Infante I, Raw AD, Ibers JA (2011). Eur J Inorg Chem 2011(28):4457–4463. https://doi.org/10.1002/ejic.201100561
Noel H, Marouille JY (1984). J Solid-State Chem 52(3):197–202. https://doi.org/10.1016/0022-4596(84)90001-X
Jin GB, Raw AD, Skanthakumar S, Haire RG, Soderholm L, Ibers JA (2010). J Solid State Chem 183(3):547–550. https://doi.org/10.1016/j.jssc.2009.12.013
Liang B, Andrews L (2002). J Phys Chem A 106(16):4038–4041. https://doi.org/10.1021/jp014301m
Liang B, Andrews L, Ismail N, Marsden CJ (2002). Inorg Chem 41(11):2811–2813. https://doi.org/10.1021/ic0255407
Armentrout PB, Beauchamp JL (1980). Chem Phys 50(1):27–36. https://doi.org/10.1016/0301-0104(80)87022-4
Lucena AF, Bandeira NAG, Pereira Cláudia CL, Gibsone JK, Marçalo J (2017). Phys Chem Chem Phys 19(16):10685–10694. https://doi.org/10.1039/c7cp01446e
Pereira CCL, Michelini MDC, Marçalo J, Gong Y, John KG (2013). Inorg Chem 52(24):14162–14167. https://doi.org/10.1021/ic4020493
Yu W, Andrews L, Wang X (2017). J Phys Chem A 121(46):8843–8855. https://doi.org/10.1021/acs.jpca.7b09454
Chen X, Li Q, Andrews L, Gong Y (2018). J Phys Chem A 122(35):7099–7106. https://doi.org/10.1021/acs.jpca.8b06810
Fu R, Lu T, Chen F (2014). Acta Phys -Chim Sin 30(4):628–639. https://doi.org/10.3866/PKU.WHXB201401211
Bader RFW (1990) Atoms in molecules: a quantum theory. Clarendon Press Oxford University, Oxford
Becke AD, Edgecombe KE (1990). J Phys Chem 92:5397–5403. https://doi.org/10.1063/1.458517
Mayer I (1985). Chem Phys Lett 979(3):270–274. https://doi.org/10.1016/0009-2614(83)80005-0
Fernandez-Ramos A, Ellingson BA, Garrett BC, Truhlar DG (2007). Rev Comput Chem 23:125–232. https://doi.org/10.1002/9780470116449.ch3
Eckart C (1930). Phys Rev 35:1303–1309. https://doi.org/10.1103/PhysRev.35.1303
Wigner E (1932). Z Phys Chem 19:203–216. https://doi.org/10.1515/zpch-1932-0120
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA, Jr, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 16, Revision B.01, Gaussian, Inc, Wallingford CT
Lee C, Yang W, Parr RG (1988). Phys Rev B 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Perdew JP, Burke K, Wang Y (1996). Phys Rev B 54:16533–16539. https://doi.org/10.1103/PhysRevB.54.16533
Krishnan R, Binkley JS, Seeger R, Pople JA (1980). J Chem Phys 72:650–654. https://doi.org/10.1063/1.438955
Blaudeau JP, McGrath MP, Curtiss LA, Radom L (1997). J Chem Phys 107:5016–5021. https://doi.org/10.1063/1.474865
Küchle W, Dolg M, Stoll H, Preuss H (1994). J Chem Phys 100(10):7535–7542. https://doi.org/10.1063/1.466847
Kashinski DO, Chase GM, Nelson RG, Di Nallo OE, Scales AN, Vanderley DL, Byrd EFC (2017). J Phys Chem A 121(11):2265–2273. https://doi.org/10.1021/acs.jpca.6b12147
Liberto GD, Conte R, Ceotto M (2018). J Chem Phys 148(10):104302–104323. https://doi.org/10.1063/1.5023155
Kreienborg NM, Merten C (2019). Phys Chem Chem Phys 21:3506–3511. https://doi.org/10.1039/C8CP02395F
Lu T, Chen F (2012). J Comput Chem 33(5):580–592. https://doi.org/10.1002/jcc.22885
Zhao J, Beckers H, Huang T, Wang X, Riedel S (2018). Inorg Chem 57(4):2218–2227. https://doi.org/10.1021/acs.inorgchem.7b03109
Xu B, Shi P, Huang T, Wang X, Andrews L (2017). J Phys Chem A 121(20):3898–3908. https://doi.org/10.1021/acs.jpca.6b12217
Manzetti S, Lu T, Behzadi H, Estrafili MD, Thi Le H, Holger V (2015). RSC Adv 5:78192–78208. https://doi.org/10.1039/C5RA17148B
Canneaux S, Bohr F, Henon E (2013). J Comput Chem 35:82–93. https://doi.org/10.1002/jcc.23470
Acknowledgments
We are very grateful to Dr. Sobereva for many helpful discussions and providing us with the Multiwfn package.
Funding
This work is supported by National Natural Science Foundation of China (NSFC) (Grant No. 11604187, No. 11647040), the Natural Science Young Foundation of Shanxi Province (Grant No. 201801D221004), Cooperation projects of Institute of Applied Physics and Computational Mathematics, and Open Fund of Key Laboratory of Advanced Reactor Engineering and Safety, Ministry of Education (Tsinghua University, China).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
See supporting information for relative energies of the stationary points on the Path A, Path B and Path C, topological properties of the charge density calculated involved in the Path C, optimized Cartesian x, y, z coordinates for the Path A and B, Path C, ESP-mapped molecular vdW surfaces of OCS molecular and structures, ELF, selected geometric parameters of stationary points on the Path A, Path B and Path C PES, and Information about reaction rate. (DOCX 1959 kb)
Rights and permissions
About this article
Cite this article
Zhao, H., Li, P., Duan, M. et al. Reaction mechanism of synthetic thorium sulfides: theoretical calculation study. J Mol Model 26, 123 (2020). https://doi.org/10.1007/s00894-020-04392-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04392-7