Abstract
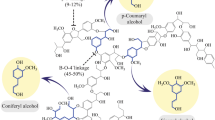
Lignin and phenolic compounds have been shown as the main recalcitrance for biomass decomposition, as they inhibit a number of lignocellulose-degrading enzymes. Understanding the inhibition mechanisms and energetic competitions with the native substrate is essential for the development of lignin resistive enzymes. In this study, atomistic detail of the size-dependent effects and binding modes of monomeric coniferyl alcohol, dimeric oligolignol, and tetrameric oligolignol made from coniferyl alcohols on the GH11 xylanase from Bacillus firmus strain K-1 was investigated by using molecular docking and atomistic molecular dynamics (MD) simulations. From the MD simulation results on the docked conformation of oligolignol binding within the “Cleft” and the “N-terminal,” changes were observed both for protein conformations and positional binding of ligands, as binding with “Thumb” regions was found for all oligolignin models. Moreover, the uniquely stable “N-terminal” binding of the coniferyl alcohol monomer had no effect on the highly fluctuated Thumb region, showing no sign of inhibitory effect, and was in good agreement with recent studies. However, the inhibitory effect of oligolignols was size dependent, as the estimated binding energy of the tetrameric oligolignol became stronger than that of the xylohexaose substrate, and the important binding residues were identified for future protein engineering attempts to enhance the lignin resistivity of GH11.

Size-dependent binding modes of coniferyl alcohol monomers (upper panels) and the dimers (lower panels). Uniquely stable “N-terminal” binding of the monomer is shown to have no effect on the binding pocket, and hence no sign of inhibition, which was in good agreement with some recent studies.







Similar content being viewed by others
References
Bian H, Wu X, Luo J, Qiao Y, Fang G, Dai H (2019) Valorization of alkaline peroxide mechanical pulp by metal chloride-assisted hydrotropic pretreatment for enzymatic saccharification and cellulose nanofibrillation. Polymers (Basel) 11(2). https://doi.org/10.3390/polym11020331
Llerena JPP et al (2019) Deposition of lignin in four species of Saccharum. Sci Rep 9(1):5877. https://doi.org/10.1038/s41598-019-42350-3
Guo F, Shi W, Sun W, Li X, Wang F, Zhao J, Qu Y (2014) Differences in the adsorption of enzymes onto lignins from diverse types of lignocellulosic biomass and the underlying mechanism. Biotechnol Biofuels 7(1):38. https://doi.org/10.1186/1754-6834-7-38
Rahikainen JL, Martin-Sampedro R, Heikkinen H, Rovio S, Marjamaa K, Tamminen T, Rojas OJ, Kruus K (2013) Inhibitory effect of lignin during cellulose bioconversion: the effect of lignin chemistry on non-productive enzyme adsorption. Bioresour Technol 133:270–278. https://doi.org/10.1016/j.biortech.2013.01.075
Pareek N, Gillgren T, Jonsson LJ (2013) Adsorption of proteins involved in hydrolysis of lignocellulose on lignins and hemicelluloses. Bioresour Technol 148:70–77. https://doi.org/10.1016/j.biortech.2013.08.121
Yoo CG, Li M, Meng X, Pu Y, Ragauskas AJ (2017) Effects of organosolv and ammonia pretreatments on lignin properties and its inhibition for enzymatic hydrolysis. Green Chem 19(8):2006–2016. https://doi.org/10.1039/c6gc03627a
Luo H, Abu-Omar MM (2017) Chemicals from lignin. In: Abraham MA (ed) Encyclopedia of sustainable technologies. Elsevier, Oxford, pp 573–585. https://doi.org/10.1016/B978-0-12-409548-9.10235-0
Cheng YS, Chen CC, Huang CH, Ko TP, Luo W, Huang JW, Liu JR, Guo RT (2014) Structural analysis of a glycoside hydrolase family 11 xylanase from Neocallimastix patriciarum: insights into the molecular basis of a thermophilic enzyme. J Biol Chem 289(16):11020–11028. https://doi.org/10.1074/jbc.M114.550905
Meng DD, Ying Y, Chen XH, Lu M, Ning K, Wang LS, Li FL (2015) Distinct roles for carbohydrate-binding modules of glycoside hydrolase 10 (GH10) and GH11 xylanases from Caldicellulosiruptor sp. strain F32 in thermostability and catalytic efficiency. Appl Environ Microbiol 81(6):2006–2014. https://doi.org/10.1128/AEM.03677-14
Anbarasan S (2016) Thermostable beta-glycosidases as processing enzymes in biorefineries. Aalto University, Aalto-yliopisto
Boukari I, O’Donohue M, Rémond C, Chabbert B (2011) Probing a family GH11 endo-β-1,4-xylanase inhibition mechanism by phenolic compounds: role of functional phenolic groups. J Mol Catal B Enzym 72(3–4):130–138. https://doi.org/10.1016/j.molcatb.2011.05.010
Silva Cde O, Aquino EN, Ricart CA, Midorikawa GE, Miller RN, Filho EX (2015) GH11 xylanase from Emericella nidulans with low sensitivity to inhibition by ethanol and lignocellulose-derived phenolic compounds. FEMS Microbiol Lett 362(13):fnv094. https://doi.org/10.1093/femsle/fnv094
Monclaro AV, Recalde GL, da Silva Jr FG, de Freitas SM, Ferreira Filho EX (2019) Xylanase from Aspergillus tamarii shows different kinetic parameters and substrate specificity in the presence of ferulic acid. Enzym Microb Technol 120:16–22. https://doi.org/10.1016/j.enzmictec.2018.09.009
Patil ND, Tanguy NR, Yan N (2016) Lignin interunit linkages and model compounds. In: Lignin in Polymer Composites. pp 27–47. https://doi.org/10.1016/b978-0-323-35565-0.00003-5
Kim S, Thiessen PA, Bolton EE, Chen J, Fu G, Gindulyte A, Han L, He J, He S, Shoemaker BA, Wang J, Yu B, Zhang J, Bryant SH (2016) PubChem substance and compound databases. Nucleic Acids Res 44(D1):D1202–D1213. https://doi.org/10.1093/nar/gkv951
Halgren TA (1996) Merck molecular force field. V. Extension of MMFF94 using experimental data, additional computational data, and empirical rules. J Comput Chem 17(5–6):616–641. https://doi.org/10.1002/(SICI)1096-987X(199604)17:5/6<616::AID-JCC5>3.0.CO;2-X
Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR (2012) Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminform 4(1):17. https://doi.org/10.1186/1758-2946-4-1719
Boonyaputthikul H, Muhammad A, Roekring S, Rattanarojpong T, Khunrae P, Sutthibutpong T (2019) Synergistic effects between the additions of a disulphide bridge and an N-terminal hydrophobic sidechain on the binding pocket tilting and enhanced Xyn11A activity. Arch Biochem Biophys 672:108068. https://doi.org/10.1016/j.abb.2019.108068
Sutthibutpong T, Rattanarojpong T, Khunrae P (2018) Effects of helix and fingertip mutations on the thermostability of xyn11A investigated by molecular dynamics simulations and enzyme activity assays. J Biomol Struct Dyn 36(15):3978–3992. https://doi.org/10.1080/07391102.2017.1404934
Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Gallo Cassarino T, Bertoni M, Bordoli L, Schwede T (2014) SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res 42(Web Server issue):W252–W258. https://doi.org/10.1093/nar/gku340
Sidhu G, Withers SG, Nguyen NT, McIntosh LP, Ziser L, Brayer GD (1999) Sugar ring distortion in the glycosyl-enzyme intermediate of a family G/11 xylanase. Biochemistry 38(17):5346–5354. https://doi.org/10.1021/bi982946f
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30(16):2785–2791. https://doi.org/10.1002/jcc.21256
Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ (1998) Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 19(14):1639–1662. https://doi.org/10.1002/(SICI)1096-987X(19981115)19:14<1639::AID-JCC10>3.0.CO;2-B
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31(2):455–461. https://doi.org/10.1002/jcc.2133426
Schmid N, Eichenberger AP, Choutko A, Riniker S, Winger M, Mark AE, van Gunsteren WF (2011) Definition and testing of the GROMOS force-field versions 54A7 and 54B7. Eur Biophys J 40(7):843. https://doi.org/10.1007/s00249-011-0700-9
Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E (2015) GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1-2:19–25. https://doi.org/10.1016/j.softx.2015.06.001
Stroet M, Caron B, Visscher KM, Geerke DP, Malde AK, Mark AE (2018) Automated Topology Builder Version 3.0: prediction of solvation free enthalpies in water and hexane. J Chem Theory Comput 14(11):5834–5845. https://doi.org/10.1021/acs.jctc.8b00768
Mark P, Nilsson L (2001) Structure and dynamics of the TIP3P, SPC, and SPC/E water models at 298 K. J Phys Chem A 105(43):9954–9960. https://doi.org/10.1021/jp003020w
Darden T, York D, Pedersen L (1993) Particle mesh Ewald: an N·log(N) method for Ewald sums in large systems. J Chem Phys 98(12):10089–10092. https://doi.org/10.1063/1.46439731
Berendsen HJC, Postma JPM, van Gunsteren WF, DiNola A, Haak JR (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81(8):3684–3690. https://doi.org/10.1063/1.448118
Martoňák R, Laio A, Parrinello M (2003) Predicting crystal structures: the Parrinello-Rahman method revisited. Phys Rev Lett 90(7):075503. https://doi.org/10.1103/PhysRevLett.90.075503
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14(1):33–38. https://doi.org/10.1016/0263-7855(96)00018-5
Laskowski RA, Swindells MB (2011) LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J Chem Inf Model 51(10):2778–2786. https://doi.org/10.1021/ci200227u
Kumari R, Kumar R, Open Source Drug Discovery C, Lynn A (2014) g_mmpbsa--a GROMACS tool for high-throughput MM-PBSA calculations. J Chem Inf Model 54(7):1951–1962. https://doi.org/10.1021/ci500020m
de Oliveira MS, da Cruz JN, Gomes Silva S, da Costa WA, de Sousa SHB, Bezerra FWF, Teixeira E, da Silva NJN, de Aguiar Andrade EH, de Jesus Chaves Neto AM, de Carvalho RN (2019) Phytochemical profile, antioxidant activity, inhibition of acetylcholinesterase and interaction mechanism of the major components of the Piper divaricatum essential oil obtained by supercritical CO2. J Supercrit Fluids 145:74–84. https://doi.org/10.1016/j.supflu.2018.12.003
Martis EAF, Coutinho EC (2019) Free energy-based methods to understand drug resistance mutations. In: structural bioinformatics: applications in preclinical drug discovery process. Chall Adv Comput Chem Phys 1–24. https://doi.org/10.1007/978-3-030-05282-9_1
Neves Cruz J, Santana de Oliveira M, Gomes Silva S, Pedro da Silva Souza Filho A, Santiago Pereira D, Lima e Lima AH, de Aguiar Andrade EH (2020) Insight into the interaction mechanism of nicotine, NNK, and NNN with cytochrome P450 2A13 based on molecular dynamics simulation. J Chem Inf Model 60(2):766–776. https://doi.org/10.1021/acs.jcim.9b00741
Silva SG, da Costa RA, de Oliveira MS, da Cruz JN, Figueiredo PLB, Brasil DSB, Nascimento LD, Chaves Neto AMJ, de Carvalho Junior RN, Andrade EHA (2019) Chemical profile of Lippia thymoides, evaluation of the acetylcholinesterase inhibitory activity of its essential oil, and molecular docking and molecular dynamics simulations. PLoS One 14(3):e0213393. https://doi.org/10.1371/journal.pone.0213393
Yang T, Wu JC, Yan C, Wang Y, Luo R, Gonzales MB, Dalby KN, Ren P (2011) Virtual screening using molecular simulations. Proteins 79(6):1940–1951. https://doi.org/10.1002/prot.23018
Ayoub AT, Elrefaiy MA, Arakawa K (2019) Computational prediction of the mode of binding of antitumor lankacidin C to tubulin. ACS Omega 4(2):4461–4471. https://doi.org/10.1021/acsomega.8b03470
Ren J, Yuan X, Li J, Lin S, Yang B, Chen C, Zhao J, Zheng W, Liao H, Yang Z, Qu Z (2020) Assessing the performance of the g_mmpbsa tools to simulate the inhibition of oseltamivir to influenza virus neuraminidase by molecular mechanics Poisson–Boltzmann surface area methods. J Chin Chem Soc 67(1):46–53. https://doi.org/10.1002/jccs.201900148
Acknowledgments
The authors would like to express their gratitude to Mr. Apichet Nguenyoung for a number of discussions regarding GH11 and lignins. The authors acknowledge the financial support provided by the King Mongkut’s University of Technology Thonburi through the “KMUTT 55th Anniversary Commemorative Fund”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 7253 kb)
Rights and permissions
About this article
Cite this article
Muhammad, A., Khunrae, P. & Sutthibutpong, T. Effects of oligolignol sizes and binding modes on a GH11 xylanase inhibition revealed by molecular modeling techniques. J Mol Model 26, 124 (2020). https://doi.org/10.1007/s00894-020-04383-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04383-8