Abstract
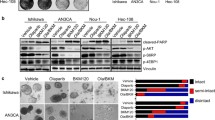
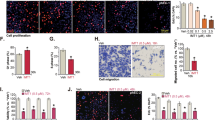
Endometrial cancer (EC) is one of the most common cancers among females worldwide. Advanced stage patients of EC have poor prognosis. Inevitable side effects and treatment tolerance of chemotherapy for EC remain to be addressed. Our results in this study showed that EC cells with higher tumor necrosis factor receptor-associated factor 4 (TRAF4) expression have lower sensitivity to poly ADP-ribose polymerase 1 (PARP1) inhibitors. Upon TRAF4 knockdown, the colony numbers of EC cells were markedly down-regulated, and the markers of DNA double-strand breakage were significantly up-regulated after the treatment of olaparib, a PARP1 inhibitor. TRAF4 knockdown reduced the phosphorylation of protein kinase B (Akt), promoted DNA double-strand breakage, and decreased levels of DNA repair related proteins, including phosphorylated-DNA-dependent protein kinase (p-DNA-PK) and RAD51 recombinase (RAD51). In addition, TRAF4′s effect on the sensitivity of EC cells to olaparib was further found to be mainly mediated by Akt phosphorylation. Moreover, in vivo results showed that TRAF4 knockdown enhanced the sensitivity of EC to PARP1 inhibitors using a mouse xenograft model. Collectively, our data suggest that combined application of TRAF4 knockdown and PARP1 inhibition can be used as a promising strategy for synthetic lethality in EC treatment.





Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366(9484):491–505. https://doi.org/10.1016/S0140-6736(05)67063-8.
Kaban A, Erdem B, Kaban I, Numanoglu C. Lymph node metastasis in early stage endometrial cancer. Eur J Gynaecol Oncol. 2018;39(3):415–21.
Braun MM, Overbeek-Wager EA, Grumbo RJ. Diagnosis and management of endometrial cancer. Am Fam Physician. 2016;93(6):468–74.
Lee YC, Lheureux S, Oza AM. Treatment strategies for endometrial cancer: current practice and perspective. Curr Opin Obstet Gynecol. 2017;29(1):47–58. https://doi.org/10.1097/GCO.0000000000000338.
Basho RK, Gilcrease M, Murthy RK, Helgason T, Karp DD, Meric-Bernstam F, et al. Targeting the PI3K/AKT/mTOR pathway for the treatment of mesenchymal triple-negative breast cancer: evidence from a phase 1 trial of mTOR inhibition in combination with liposomal doxorubicin and bevacizumab. JAMA Oncol. 2017;3(4):509–15. https://doi.org/10.1001/jamaoncol.2016.5281.
Bradley JR, Pober JS. Tumor necrosis factor receptor-associated factors (TRAFs). Oncogene. 2001;20(44):6482–91. https://doi.org/10.1038/sj.onc.1204788.
Kedinger V, Rio MC. TRAF4, the unique family member. Adv Exp Med Biol. 2007;597:60–71. https://doi.org/10.1007/978-0-387-70630-6_5.
Shiels H, Li X, Schumacker PT, Maltepe E, Padrid PA, Sperling A, et al. TRAF4 deficiency leads to tracheal malformation with resulting alterations in air flow to the lungs. Am J Pathol. 2000;157(2):679–88. https://doi.org/10.1016/S0002-9440(10)64578-6.
Regnier CH, Masson R, Kedinger V, Textoris J, Stoll I, Chenard MP, et al. Impaired neural tube closure, axial skeleton malformations, and tracheal ring disruption in TRAF4-deficient mice. Proc Natl Acad Sci USA. 2002;99(8):5585–90. https://doi.org/10.1073/pnas.052124799.
Li W, Peng C, Lee MH, Lim D, Zhu F, Fu Y, et al. TRAF4 is a critical molecule for Akt activation in lung cancer. Can Res. 2013;73(23):6938–50. https://doi.org/10.1158/0008-5472.Can-13-0913.
Singh R, Karri D, Shen H, Shao JY, Dasgupta S, Huang SX, et al. TRAF4-mediated ubiquitination of NGF receptor TrkA regulates prostate cancer metastasis. J Clin Invest. 2018;128(7):3129–43. https://doi.org/10.1172/Jci96060.
Zhu L, Zhang S, Huan X, Mei Y, Yang H. Down-regulation of TRAF4 targeting RSK4 inhibits proliferation, invasion and metastasis in breast cancer xenografts. Biochem Biophys Res Commun. 2018;500(3):810–6. https://doi.org/10.1016/j.bbrc.2018.04.164.
Yang K, Wang F, Han JJ. TRAF4 promotes the growth and invasion of colon cancer through the Wnt/beta-catenin pathway. Int J Clin Exp Pathol. 2015;8(2):1419–26.
Yao W, Wang X, Cai Q, Gao S, Wang J, Zhang P. TRAF4 enhances osteosarcoma cell proliferation and invasion by Akt signaling pathway. Oncol Res. 2014;22(1):21–8. https://doi.org/10.3727/096504014X14077751730351.
Xie PM, Wang XL, Kong M, Bai XY, Jiang T. TRAF4 promotes endometrial cancer cell growth and migration by activation of PI3K/AKT/Oct4 signaling. Exp Mol Pathol. 2019;108:9–16. https://doi.org/10.1016/j.yexmp.2019.03.003.
Herceg Z, Wang ZQ. Functions of poly(ADP-ribose) polymerase (PARP) in DNA repair, genomic integrity and cell death. Mutat Res. 2001;477(1–2):97–110. https://doi.org/10.1016/S0027-5107(01)00111-7.
Ratnam K, Low JA. Current development of clinical inhibitors of poly (ADP-ribose) polymerase in oncology. Clin Cancer Res. 2007;13(5):1383–8. https://doi.org/10.1158/1078-0432.Ccr-06-2260.
Michels J, Vitale I, Galluzzi L, Adam J, Olaussen KA, Kepp O, et al. Cisplatin resistance associated with PARP hyperactivation. Can Res. 2013;73(7):2271–80. https://doi.org/10.1158/0008-5472.Can-12-3000.
Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355(6330):1152–8. https://doi.org/10.1126/science.aam7344.
Ali R, Al-Kawaz A, Toss MS, Green AR, Miligy IM, Mesquita KA, et al. Targeting PARP1 in XRCC1-deficient sporadic invasive breast cancer or preinvasive ductal carcinoma in situ induces synthetic lethality and chemoprevention. Can Res. 2018;78(24):6818–27. https://doi.org/10.1158/0008-5472.Can-18-0633.
Bi FF, Li D, Yang Q. Hypomethylation of ETS transcription factor binding sites and upregulation of PARP1 expression in endometrial cancer. Biomed Res Int. 2013. https://doi.org/10.1155/2013/946268.
Zhu D, Sun C, Qian X. MST1 suppresses viability and promotes apoptosis of glioma cells via upregulating SIRT6 expression. J Integr Neurosci. 2019;18(2):117–26. https://doi.org/10.31083/j.jin.2019.02.122.
Kastenmayer RJ, Moore RM, Bright AL, Torres-Cruz R, Elkins WR. Select agent and toxin regulations: beyond the eight edition of the guide for the care and use of laboratory animals. J Am Assoc Lab Anim. 2012;51(3):333–8.
Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase-AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501. https://doi.org/10.1038/nrc839.
Liu J, Eckert MA, Harada BT, Liu SM, Lu ZK, Yu KK, et al. m(6)A mRNA methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer. Nat Cell Biol. 2018;20(9):1074. https://doi.org/10.1038/s41556-018-0174-4.
Toulany M, Lee KJ, Fattah KR, Lin YF, Fehrenbacher B, Schaller M, et al. Akt promotes post-irradiation survival of human tumor cells through initiation, progression, and termination of DNA-PKcs-dependent DNA double-strand break repair. Mol Cancer Res. 2012;10(7):945–57. https://doi.org/10.1158/1541-7786.Mcr-11-0592.
Mueck K, Rebholz S, Harati MD, Rodemann HP, Toulany M. Akt1 stimulates homologous recombination repair of DNA double-strand breaks in a Rad51-dependent manner. Int J Mol Sci. 2017. https://doi.org/10.3390/ijms18112473.
Liu KR, Wu XL, Zang X, Huang ZJ, Lin ZY, Tan WL, et al. TRAF4 Regulates migration, invasion, and epithelial-mesenchymal transition via PI3K/AKT signaling in hepatocellular carcinoma. Oncol Res. 2017;25(8):1329–40. https://doi.org/10.3727/096504017x14876227286564.
Zhang L, Li XR, Yang WL, Jiang XK, Li N. TRAF4 promotes tumorigenesis of breast cancer through activation of Akt. Oncol Rep. 2014;32(3):1312–8. https://doi.org/10.3892/or.2014.3304.
Wang AL, Wang J, Ren HY, Yang F, Sun LL, Diao KX, et al. TRAF4 participates in Wnt/beta-catenin signaling in breast cancer by upregulating beta-catenin and mediating its translocation to the nucleus. Mol Cell Biochem. 2014;395(1–2):211–9. https://doi.org/10.1007/s11010-014-2127-y.
Zhang Y, Zhang R, Sui R, Chen Y, Liang H, Shi J, et al. MicroRNA-374a governs aggressive cell behaviors of glioma by targeting prokineticin 2. Technol Cancer Res Treat. 2018;18:1533033818821401.
Liu YJ, Duan NQ, Duan SB. MiR-29a inhibits glioma tumorigenesis through a negative feedback loop of TRAF4/Akt Signaling. Biomed Res Int. 2018. https://doi.org/10.1155/2018/2461363.
Chen TJ, Gao F, Feng SF, Yang T, Chen MW. MicroRNA-370 inhibits the progression of non-small cell lung cancer by downregulating oncogene TRAF4. Oncol Rep. 2015;34(1):461–8. https://doi.org/10.3892/or.2015.3978.
Meerson A, Yehuda H. Leptin and insulin up-regulate miR-4443 to suppress NCOA1 and TRAF4, and decrease the invasiveness of human colon cancer cells. BMC Cancer. 2016. https://doi.org/10.1186/s12885-016-2938-1.
Qi YC, Zhou YL, Chen XX, Ye L, Zhang QW, Huang FJ, et al. MicroRNA-4443 causes CD4+T cells dysfunction by targeting TNFR-associated factor 4 in Graves' disease. Front Immunol. 2017. https://doi.org/10.3389/fimmu.2017.01440.
Wang JC, Quan XY, Peng DT, Hu G. Long non-coding RNA DLEU1 promotes cell proliferation of glioblastoma multiforme. Mol Med Rep. 2019;20(2):1873–82. https://doi.org/10.3892/mmr.2019.10428.
Galaal K, Al Moundhri M, Bryant A, Lopes AD, Lawrie TA. Adjuvant chemotherapy for advanced endometrial cancer. Cochrane Database Syst Rev. 2014. https://doi.org/10.1002/14651858.CD010681.pub2.
Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7(7):517–28. https://doi.org/10.1038/nrm1963.
Weigelt B, Warne PH, Lambros MB, Reis-Filho JS, Downward J. PI3K pathway dependencies in endometrioid endometrial cancer cell lines. Clin Cancer Res. 2013;19(13):3533–44. https://doi.org/10.1158/1078-0432.Ccr-12-3815.
Philip CA, Laskov I, Beauchamp MC, Marques M, Amin O, Bitharas J, et al. Inhibition of PI3K-AKT-mTOR pathway sensitizes endometrial cancer cell lines to PARP inhibitors. BMC Cancer. 2017. https://doi.org/10.1186/s12885-017-3639-0.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
LLT and CYZ conceived and designed the experiments, MW analyzed and interpreted the results of the experiments, LJ performed the experiments.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that there were no competing interests, and all authors confirmed its accuracy.
Ethics approval and consent to participate
The animal study was approved by the Ethics Committee of The Third Affiliated Hospital of Southern Medical University. Experiments were operated according to the Guidelines for the Care and Use of Laboratory Animals published by the National Institutes of Health.
Patient consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tang, L., Wang, M., Jiang, L. et al. TRAF4 knockdown triggers synergistic lethality with simultaneous PARP1 inhibition in endometrial cancer. Human Cell 33, 801–809 (2020). https://doi.org/10.1007/s13577-020-00363-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-020-00363-5