Abstract
Oil extraction and biodiesel production process produce a massive amount of by-products like Jatropha press cake (JPC) and crude glycerol (CG), which could be used as a potential substrate for methane production. However, the higher lignocellulosic and nitrogen content in the JPC act as a recalcitrant and inhibitor, respectivly, for microbes that are involved in the anaerobic digestion (AD) process. Therefore, the present study aimed to enhance the methane yield of JPC by optimizing the alkaline pretreatment and co-digestion process conditions. The effects of NaOH concentration, incubation temperature, and retention time on methane and soluble chemical oxygen demand (sCOD) yields were evaluated and modeled by employing a response surface methodology coupled with central composite design (RSM-CCD). Moreover, a series of batch experiments with various feedstock concentrations (FCs) were tested to investigate the methane yield of JPC when co-digested with CG at different levels. The methane yields of all pretreated samples were significantly higher when compared with these of the untreated JPC. Pretreating the JPC using 7.32% NaOH at 35.86 °C for 54.05 h was the optimum conditions for maximum methane increment of 40.23% (353.90 mL g−1 VS), while co-digesting 2% CG with JPC at 2 g VS L−1 FC enhanced the methane yield by 28.9% (325.47 mL g−1 VS). Thus, the methane yield of JPC was effectively increased by alkaline pretreatment and co-digesting with CG. However, the alkaline pretreatment was relatively more effective compared with the co-digestion process.
Similar content being viewed by others
1 Introduction
The demand for fuel energy sources and their actual consumption across the globe are increasing drastically [1]. Fossil fuel resources supplied almost 87% of the global energy demand [2]. However, three-fourths of the world’s poor households consumed only 10% of the worldwide energy supply [3]. Most of these poor households are living in net oil-importing Sub-Sahara Africa, where Ethiopia is ranked at the bottom of the energy poverty index [4]. In Ethiopia, 91% of the energy demand is satisfied by the local available traditional biomass resources [5]. However, the utilization of these conventional energy sources has resulted in severe environmental problems like forest degradation and greenhouse gas emissions. Furthermore, the entire transport energy requirement of Ethiopia is dependent on imported petroleum, with the cost of 65–80% of the total export earnings [6]. For instance, in 2018/2019 alone, 3.99 million metric tons of petroleum products were imported; and the expense of this import was more than 2.4 billion US dollars [7]. The costs associated with port rent, along with petroleum price and fluctuation, have severely affected the trade balances of the country. As a result, the Ethiopian government has launched a strategy in 2007 to assist the production and utilization of biofuels (biodiesel, bioethanol, and biogas) from different non-edible oil seeds and agro-industrial and municipal solid organic wastes [8].
Jatropha curcas L. (Jatropha hereafter), castor bean (Ricinus communis), Croton (Croton macrostachyus), and Moringa (Moringa stenopetala) plant seeds have been identified as promising non-edible energy plants for biodiesel production [9]. Jatropha seed is characterized by high oil content [10]. The oil is dominated by monounsaturated and polyunsaturated fatty acids [11]. Although the seeds are suitable for biodiesel production, 65–70% of them reported being disposed of as Jatropha press cake (JPC) during oil extraction [12], while 100 kg of biodiesel production generates 10–14 kg of crude glycerol (CG) [13]. Considering the availability of adequate Jatropha plantation in Ethiopia [4] and higher seed production per ha [14], biodiesel production would generate a substantial quantity of JPC (10.5–11.4 million tons) and CG (0.61–0.70 million tons) per year.
CG can be purified into high grade for various applications in cosmetics and pharmaceuticals. However, the high purification cost hinders its use in such industries and usually considered organic waste [2]. While JPC contains toxic chemicals like phorbol ester and curcin [15], thus, the cake could not be directly used as animal feed or organic fertilizer [16]. As a result, managing and controlling these organic wastes become the most challenging issue for most biodiesel producers. Detoxification and anaerobic digestion (AD) were identified as promising pretreatment methods for utilizing JPC for animal feed and organic fertilizer, respectively. With regard to this, studies have tried to investigate the biochemical methane potential (BMP) of JPC [17, 18]. However, JPC contains a considerable amount of seed husks, which are enriched with lignocellulosic materials. Liang et al. [19] showed that JPC contains 19% lignin and 27% carbohydrates, while Kumar et al. [20] measured 14% and 28% cellulose and hemicellulose, respectively. Furthermore, the carbon-to-nitrogen ratio (C/N) of JPC was reported to be 9:1 [16], which is significantly lower compared with the optimum ratio of 20:1–30:1 [21]. The digestibility of lignocellulosic biomasses for methane production is limited mainly due to the rate-limiting hydrolysis stage caused by the complex cellulose-hemicellulose-lignin networks [22]. Higher nitrogen content may cause inhibition of the methanogenic activities [23] and subsequently leads to process collapse due to the ammonia accumulation [18]. In contrast, CG is very rich in carbon, which means the mono-digestion of CG for biogas production is impossible due to nitrogen deficiency for maintaining the microbial biomass growth [2].
Pretreating the JPC using proper techniques can improve the anaerobic degradation process by bringing a structural change. The structural change would help the microbes to convert the carbohydrate polymers into simple fermentable sugars [24], whereas, co-digestion could improve the methane yield by optimizing the required C/N ratio [2, 25]. Therefore, various techniques such as mechanical [26], thermochemical [24], and steam explosion [27, 28] pretreatments were commonly used for pretreating lignocellulosic biomasses. Furthermore, the biodegradability of feedstocks with lower C/N ratio could be improved by co-digestion, solid-state AD, bio-augmentation, or nutrient supplementation [21, 23].
In the present study, alkaline pretreatment and co-digestion process were selected as a potential technique for enhancing the methane yields of JPC due to various positive characteristics compared with other pretreatment methods. Briefly, thermo-acidic pretreatments are corrosive and cause degradation of carbohydrates and lignin [29]. Biological pretreatments are expensive and time-consuming due to the slow processes, and finding a specific enzyme is complicated [21, 26, 30, 31]. Physical pretreatments are energy-intensive processes [21, 26]. The alkaline pretreatment may increase the pH of the feedstock due to the residual chemical after the pretreatment [32]. However, the remaining chemical is useful since the next step, the AD, requires an alkaline addition for controlling pH drop due to volatile fatty acid (VFA) accumulation. Moreover, alkaline pretreatment is highly effective in lignin removal by cleaving the lignin-carbohydrate linkages with minimal carbohydrate degradation [21].
Among different alkaline chemicals, NaOH is effective for enhancing the biogas yield of lignocellulose biomasses [21]. However, the efficiency of alkaline pretreatments is affected mainly by chemical concentration, temperature, and retention time [33,34,35]. The effect of these process variables in the alkaline pretreatment was studied by varying one variable at a time but keeping the other variables into a constant value. However, the synergistic effects from simultaneous changes of more than one process variable were not widely investigated. Failure in optimizing the effects of individual and interactive process variables would lead to insufficient hydrolysis of lignocellulosic materials or AD process inhibition due to the accumulation of degraded products [36]. To the best of our knowledge, no reports are found on the optimum condition of alkaline pretreatment, and the amount of CG that should be added for enhancing the methane yield of JPC. Thus, the present study was initiated to examine and model the effect of NaOH concentration, incubation temperature, and retention time in the alkaline pretreatment on the methane and soluble chemical oxygen demand (sCOD) yield of JPC.
2 Materials and methods
2.1 Source of raw materials
The JPC sample was kindly provided by YME Design and Manufacture Company located at Hawassa, Ethiopia. At the time of supply, the company exploded the oil from the Jatropha seed using a hydraulic press machine. The fresh JPC was dried at room temperature (27 °C) until its moisture content reduced to 10% on the dry weight basis. The dried JPC was then transferred into airtight zipped plastic and stored at 4 °C until further processing. The bacterial inoculum prepared from cow manure was collected from a biogas laboratory operated under mesophilic conditions. The dominant types of microbes living in the manure-based inoculum were identified by Ozbayra et al. [37]. Firmicutes and Bacteroidetes accounted for about 46% and 36% of the bacterial community, respectively, while the methanogenic community was dominated by methanomicrobia (65%), methanobacteria (25%), and methanoplasma (8%) archaea. The analytical grade of NaOH for the alkaline pretreatment was purchased from VWR, Oslo, Norway. The CG that co-digested with JPC was purchased from biodiesel producer company, Perstorp AB, located in Sweden.
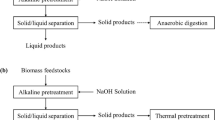
2.2 Co-digestion of Jatropha press cake with crude glycerol
The BMP of JPC that co-digested with CG at various levels (0, 1, 2, 3, 4 wt%, on VS basis) was investigated by following a completely randomized design [2]. The actual experimental layout for the AD experiment is shown in Table 1, and each treatment was duplicated into a total of 30 batch reactors.
2.3 Alkaline pretreatment
The alkaline pretreatment was conducted by following the method described by Zhu et al. [38], with some modifications. Accordingly, JPC was allowed to pass through a sieve with a mesh size of ≤ 2.5 mm [39]. Then, 100 g of sun-dried sample (with 8% moisture content) was transferred into 1-L beakers that contain 2.64%, 4%, 6%, 8%, and 9.36% of NaOH, based on the dry weight of JPC. The samples were then adjusted into a moisture content of 82% by adding the required amount of tap water (Eq. (1)) [40]. After adding the water, all samples were gently stirred with glass road to homogenize the mixture. Then after, all beakers were covered with plastic films and fastened with plastic rings and incubated at 26.6 °C, 30, 35, 40, and 43.4 °C for 7.68, 24, 48, 72, and 88.82 h. Following the subsequent pretreatments, a subsample was taken from individual treatment and centrifuged at 6000 rpm for 20 min to separate the solid and liquid fraction for sCOD analysis. Finally, the solid/liquid mixture and liquid fractions of the samples were immediately transferred into zipped plastic bags and 20-mL vials, respectively. Both samples were stored in the freezer until the actual biogas production and sCOD analysis were started. For the control experiment, 100 g of JPC was diluted with the same amount of water without alkaline and incubated under room temperature for the maximum retention time of 88.82 h [29].
where MC is moisture content and JPC is Jatropha press cake.
2.4 Biochemical methane potential assays
The effects of alkaline pretreatment and co-digestion of CG with JPC on the BMP were investigated using 57 and 32-batch reactors, respectively. The total and working volume of the batch reactors for alkaline samples were 122 and 70 mL, respectively, while the bigger batch reactors with 530 and 350 mL of total and working volume, respectively, were used in the co-digestion process. The main reason for preferring the bigger batch reactor in the co-digestion process was the limited working volume from the smaller reactor for adding the required amount of CG. In contrast, the smaller reactors were used for the alkaline-pretreated samples due to the inoculum shortage. Moreover, the authors assumed that the effect of volume variation on methane yield is minimal since the inoculum-to-substrate ratio (ISR), incubation temperature, agitation rate, and reactor configuration were the same for all batch reactors.
The manure-based inoculum was further incubated at 37 ± 0.5 °C under the anaerobic condition for 1 week, which helps the microbes to assimilate the environment and reduce the endogenous biogas production. Following the pre-incubation, the prepared reactors were filled with the required amount of inoculum (5 g VS L−1); then, 3.34 g VS L−1 of untreated or pretreated JPC was added into all batch reactors. Thus, in this experiment, the ISR was kept to 1.5 on a VS basis [27]. However, the amount of VS added into the bigger batch reactor (530 mL) depends on the predefined FCs, i.e., 2, 5, and 7 g VS L−1. The respective working volumes of the reactors were then adjusted by diluting the inoculum and substrate mixture with tap water. For blank and positive control experiments, the same amount of inoculum was poured to each bottle, and tap water was added for required working volume: 1.5 g L−1 pure cellulose (C6H10O5) n < 20 μm was added into the bottles reserved for positive control. For alkaline-pretreated JPC, triplicated bottles were prepared for samples pretreated under the corner and axial (star) points, whereas six bottles were prepared for samples pretreated under the center points (Table 2). The batch reactors arranged for co-digestion experiments were duplicated, and a total of 30 bottles ware utilized. All batch reactors were closed with a rubber stopper and aluminum crimps and then purged with pure nitrogen using a syringe for 5 min to make an anaerobic environment [41]. Finally, the reactors were incubated at 37 ± 0.5 °C and continuously centrifuged to 90 rpm. The methane yield increment (MYI) due to the alkaline pretreatments and the co-digestion processes were estimated using Eq. (2) [42].
where MYI is methane yield increment.
2.5 Experimental design
The alkaline pretreatments were carried out for each combination of all variables (NaOH concentration, incubation temperature, and retention time) using RSM-CCD, and all pretreatments were carried out in random orders. A total of 20 treatments that containing eight factorial points, six axial points, and six replicates for the center value were applied. The values estimated from center points were used to determine the experimental error. The coded and real values were calculated using Design-Expert version 12 (State Ease Inc., Statistics Made Easy, Minneapolis, MN, USA). The lower and upper values of each factor (Table 2) were determined following the methods of Zheng et al. [43]. The data obtained from AD and hydrolysates were used to develop a second-order polynomial equation that relates the responses to the process variables (Eq. (3)). Both the experimental design and statistical analysis were also done using the same software. Furthermore, the second-order polynomial equation from RSM-CCD was used to define the optimum value of each process variable that could maximize the methane and sCOD yields. The linear, interactive, and quadratic effect of temperature, NaOH concentration, and retention time was evaluated using a multivariate analysis of variance (ANOVA). The contour and 3D response surface plots were drawn to depict the interactive effect between two process variables while keeping the third variable into its center value. Values for multiple R2, adj. R2, and pred. R2 were used to evaluate the fitted model.
where Y is the response (sCOD or methane yield); X1, X2, and X3 are the coded independent variables; and β0, β1, β2 and β3, β11, β22, β33, β12, β13, and β23 are the model coefficients calculated from the experimental value.
2.6 Analytical method
2.6.1 Substrate analysis
The total solid (TS) and volatile solid (VS) contents of inoculum and JPC were determined based on the APHA standards [44]. The carbon, nitrogen, and hydrogen contents were estimated using an elemental analyzer (Thermo Flash 2000) from soil laboratory located at Norwegian University of Life Sciences, Ås, Norway. The crude protein content of JPC was estimated from its nitrogen content [45]. Karl Fisher titration was used for determining the moisture content of CG [45], and the TS was calculated by considering the moisture content. The VS content of CG was assumed to be the same with the TS value as nothing was left in the crucible when it was ignited at 550 °C for 2 h. The sCOD values for each treatment were estimated following DIN ISO 15705, which is analogs to EPA 4104 and APHA 5220 D [42]. Merck Spectroquant® COD kits with a measuring range of 500–10,000 mg L−1 were used in the analysis of the sCOD yields. The caloric value and crude fat content of the dried JPC were determined using a Bomb Calorimeter (IKAR C 200) [42] and conventional Soxhlet extraction method, respectively [18].
2.6.2 Biogas analysis
The BMP of both the alkaline pretreated and co-digested JPC with CG were regularly monitored for 61 and 56 days, respectively, until the last methane production in all flasks was less than 2% of the total amount. Briefly, the biogas pressure was measured using a digital manometer (GMH 3161 Reisinger Electronics, Germany), and the concentration of CO2 and CH4 in the biogas was determined using gas chromatography (3000 Micro GC, Agilent Technologies, USA), equipped with a thermal conductivity detector (TCD) using helium as a carrier gas [42]. After the successive biogas measurements, the excessive pressure was released by inserting the needle into the rubber stopper. All measured gas volumes were reported at 273 K and 101.3 kPa using Eq. (4), and the specific and cumulative methane volume was calculated using the normalized methane concentrations. The endogenous methane value produced from the blank controls (inoculum) was subtracted from the total methane yield. Besides CO2 and CH4, the biological degradation of organic matter could generate hydrogen gas. However, considering its small fraction (5–10%) when compared with the total biogas yield [46], this study did not measure the hydrogen concentration during the AD process.
where Vb is the volume of biogas (L) at the standard condition of 273 K (0 °C), 1 atm total pressure; Po is 1 atm; n is the number of moles; R is the ideal gas constant; V is the volume of headspace (L); T is the temperature in the incubator room (310 k); and dp is the overpressure measured in the bottle (atm) (1013.25 mbar = 1 atm).
2.6.3 Energy balance and economic feasibility
Energy balance and benefit-cost analysis are essential tools for examining the economic viability of various pretreatment techniques applied for enhancing the biogas production potential lignocellulosic material. Although all alkaline and co-digestion processes improved the methane yield of JPC, energy balance and cost analysis were done only for the optimum conditions identified for higher methane yields by using Eqs. (5) and (6) [47]. The benefit-cost ratio (BCR) analysis was performed based on the following assumptions: (1) the average market price of NaOH is assumed to be 412 € t−1 [48] and (2) the estimated cost of methane sale is 0.57 € m−3 [47]. Moreover, the optimum temperature (35.86 °C) identified in the alkaline pretreatment is nearly the same as the maximum ambient temperature (33 °C) of Hawassa City, Ethiopia, where the JPC sample was brought. Thus, we assumed that no heat energy input was needed for this alkaline pretreatment.
where Eo is energy output (kJ kg−1 VS); Ei is energy input (kJ kg−1 NaOH); ENaOH energy needed for 1-kg alkaline production (7 MJ kg−1); ∆p is net methane yield increment (m3 g−1 VS); ξ is methane heating value (35.8 kJ L−1); ɳ is methane energy conversion efficiency (90%), and n is the amount of NaOH needed in the pretreatment process (kg).
2.6.4 Statistical analysis
The interactive effect between FC and CG levels on the biogas and methane yield of JPC was tested using a two-way ANOVA via R software (version 3.6.2), while the linear, interactive, and quadratic effects of NaOH concentration (X1), incubation temperature (X2), and retention time (X3) were analyzed using Design-Experts (version 12) as discussed in Section 2.5. All ANOVA was carried out using Tukey’s test. Thus, there were statistically significant variations at p ≤ 0.05 between mean with different letter groups, or the mean differences were considered statistically significant if the p value is ≤ 0.05.
3 Results and discussion
3.1 Substrate compassion
The physical and chemical constituents of JPC, CG, and inoculum characterized using the methods described in Section 2.6.1 are shown in Table 3.
3.2 Impact of feedstock concentration and CG level on BMP of JPC
Various studies indicate that FC has a detrimental effect on the AD process and the subsequent methane recovery process [49,50,51]. Moreover, the higher protein, lipid, and long-chain free fatty acid concentration present in the JPC could also inhibit the activity of microbes in the digester unless the steady-state condition is maintained. In the present study, the feedstock concentration (FC) is defined as the amount of JPC and CG in VS bases added into the working volume of the batch digester (g VS L−1). Significant variations in cumulative and specific methane yields were observed among treatments operated under different FCs (2, 5, and 7 g VS L−1) and CG levels (0, 1, 2, 3, 4%). Most of the batch digesters run without CG produced lesser biogas and methane when compared with the treatments containing both CG and JPC (Table 4). The lower methane yield might be attributed to the higher content of lignocellulosic materials along with lower C/N ratios. The availability of higher nitrogen in the JPC could generate non-ionized and/or ammonium ion in the AD process [52]. Then, the ammonium reacts with water and forms ammonium hydroxide (NH4OH), which alkalizes the bulk solution of the digester. The alkalized bulk solution could inhibit the anaerobic process by affecting the activity of Archaea methanogenesis [53]. Moreover, variation in trends and peaks of daily methane yield also noted among treatments (Fig. 1a, b, and c). Co-digesting 2% CG with JPC at 2 g VS L−1 FC showed a fast degradation rate, which needed only 3 days to attain the maximum methane peak value (48.9 mL g−1 VS day−1) when compared with all other treatments (Fig. 1a).
A two-way ANOVA and Tukey post hoc test were conducted to investigate the statistical variation in biogas and methane yields among digesters operated at various co-digestion conditions. The smaller p value (< 2.2 × 10−16) of the ANOVA model indicates a statistically significant variation among anaerobic digesters in estimated cumulative biogas and methane yields (Table 4). The cumulative methane yield of JPC slightly declined as increasing of both the FC and CG levels (Fig. 1d). The average cumulative methane yield of digester run by adding various concentrations of CG to JPC under 2 g VS L−1 FC ranging between 252.41 to 325.47 mL g−1 VS, while the methane yield obtained from 5 g VS L−1 and 7 g VS L−1 FCs varied from 240.11 to 275.69 and 165.87 to 254.19 mL g−1 VS, respectively, depending upon the amount of added CG levels.
The highest cumulative methane yield (325.47 mL g−1 VS) was obtained from digester working under 2 g VS L−1 that contains 2% CG, while less methane yield (165.87 g−1 VS) was recorded from digester operated under 7 g VS L−1 and enriched with 4% CG. The methane increment recorded from the former treatment was 28.9% when compared with the samples containing no CG at the same FC. However, the methane yield obtained from treatment that co-digested 4% CG with JPC at 7 g VS L−1 FC declined by 96.23% when compared with the maximum methane yield. Similar results were reported by Schwingel et al. [2], in which the biogas and methane yield from laying hen waste were strongly dependent on the amount of added CG. Oliveira et al. [54] noted that co-digesting 2% CG with sargassum resulted in 18% more methane as compared with the control treatment, while Schwingel et al. [2] showed that co-digesting 2.6% glycerol with laying hen manure produced 21.83% more methane than the manure without glycerol. Veroneze et al. [55] indicated that inhibition of methane production from swine manure when more than 5% of glycerol was added.
The increment in methane yield might be attributed to the higher biodegradability and enhancement of the C/N ratio due to the added CG. In contrast, the lower methane yield might be associated with organic overloading, fast production of VFAs, or acidification of the digester [43]. The rapid production of VFA due to the fast hydrolysis of CG may inhibit the methanogenesis bacteria [38]; however, its effect seemed small since variation in methane concentrations among reactors was not statistically significant. Organic overloading could stimulate the microbial production of biosurfactants and mostly resulted in partial degradation of organic matter [56], which subsequently causes lower methane yield (Fig. 1d). The VS content of the digestate after 61 days of AD was higher for batch reactors operated at higher FC. The effect of overloading on methane yield could be higher since JPC contains less biodegradable protein [50]. Moreover, stable small bubbles (foams) were observed in the batch reactors run at higher FC. The foaming incidence might be associated with the surfactant nature of protein and lipid available in the JPC [57]. Thus, the foaming incidence could reduce the methane yield by producing a gas-liquid film at the top of the digester.
Variation in average cumulative methane yield was also investigated for samples with the same FC but contained different levels of CG using a single-factor ANOVA. The p values of digesters working under 2, 5, and 7 g VS L−1 FCs were 0.02, 0.18, and < 0.01, respectively, which indicate that the effects of added CG levels were statistically significant at lower and higher FCs; however, their effects were negligible at 5 g VS L−1 FC. Besides the cumulative methane yield, the average methane concentrations were calculated for all samples and the values ranging from 70.7 to 73.9%. The ANOVA model showed an insignificant variation in methane concentration among treatments. Therefore, considering the economic gain of using lower FC for higher cumulative methane production, co-digesting 2% CG with JPC at 2 g VS L−1 FC was identified as the optimum condition for higher methane production. The optimum condition obtained in the present study was comparable with conditions suggested by Haitl et al. [58]; however, relatively higher methane yield (1058.9 mL g−1 VS) was reported by co-digesting 2.5% CG with hen laying manure [2].
3.3 Modeling the degree of solubilization (sCOD)
The effect of alkaline pretreatment on the compositional change of JPC was assessed using the sCOD yields as a primary indicator [41]. A regression model was fitted to determine the optimum values of the selected process variables for higher sCOD production. By referring Eq. (3), the second-order polynomial equation obtained from the experimental data was taken in terms of coded and actual value to evaluate the correlation between predictors (X1, X2, and X3) and the response (sCOD) variables (Eqs. (7) and (8)). Thus, the liner, interactive, and quadratic effects of X1, X2, and X3 on the degree of solubilization were investigated using RSM-CCD. As shown in Eq. (7), all coded linear and two interaction terms had a positive effect, while all quadratic terms and the interaction between X2 and X3 adversely affected the degree of solubilization.
The adj. R2 value of the quadratic model (0.81) was higher as compared with the values estimated for linear (0.68) and two-factor interaction (0.63) models; hence, the second-order polynomial equation was the preferred model in this study [59]. The strong correlation between experimental and model-predicted sCOD values further exhibited the suitability of the model (Fig. 2b). This value lies between ± 4.3, in which all estimated values were close to the model-predicted value. The value for the coefficient of determination (R2) was 0.90, which assured the applicability of the model for investigating the effect of these process variables in the alkaline pretreatment process. Furthermore, the p value > 0.05 indicates that there was no evidence on the model’s lack of fit relative to the pure error, which further elaborated the strength and adequacy of the model to depict the correlation between predictor and response variables.
The multivariate ANOVA for the second-order polynomial equation was also done to test the strength of the model. The results suggested that the second-order polynomial equation could better describe the relationship between sCOD productions and operational variables. As shown in Table 5, the p values ≤ 0.05 indicate the significant linear impact of NaOH concentration (X1) and retention time (X3), as well as the quadratic effects from the same variables (X12 and X32). Even though the p value of temperature was slightly greater than 0.05, it was included in the model by considering as important process variables in the solubilization of the JPC. This finding was in agreement with the report by Monlau et al. [60].
3.3.1 Effect of independent processing parameters on the degree of solubilization
Depending on the impact of various process variables, the sCOD yield increments ranging between 200 to 488% compared with the untreated JPC (Table 6). The increased sCOD yields were comparable with the reported value for greenhouse crop waste after the alkaline H2O2 pretreatment [61]. The effect of three independent process variables on the sCOD yield of JPC is shown in Fig. 3b, d, and f. The impact of individual process variables on the degree of solubilization was examined by maintaining the other two variables into their center value.
The lowest sCOD yield (82 g L−1) was obtained when the JPC was pretreated by adding 6% of NaOH and incubated at 35 °C for 7.6 h. In comparison, the highest value of 161 g L−1 was recorded at the same NaOH concentration and incubation temperature but retained for 88.4 h (Table 6). Figure 3b shows that the sCOD yield from JPC was increased by 60% when the NaOH concentration increased from 2.64 to 6% by keeping the incubation temperature and retention time to their center value. However, with a further increase in NaOH concentration from 6 to 9.36%, the rate of sCOD yield increment showed a declining trend; only 6.85% improvement was observed. The catalyst (NaOH) was highly effective in attacking the binding bond between lignin and hemicellulose [62]. Moreover, NaOH is also effective for cleaving the ester and carbon-to-carbon bonds in lignin molecules (ferulic acid) and resulted in the release of soluble compounds from carbohydrate and lignin components. In the alkaline pretreatment, the NaOH would dissociate into two Na+ and OH− ions, and as the OH− concentration increases, the rate of hydrolysis increases consequently [63].
The incubation temperature at all levels slightly increased the degree of solubilization from JPC (Fig. 3d). The sCOD yield increased by 11.11% when the incubation temperature increased from 26.6 to 43.4 °C, which implies that the alkaline pretreatment was effective at mild temperatures. However, the effect of temperature on sCOD yield was relatively lower when compared with the other pretreatment variables. The weaker impact of temperature on the degree of solubilization might be associated with the lower temperature ranges (26.60–43.40 °C) applied in the pretreatment processes. In the same way, the effect of retention time on sCOD yield was highly significant in its lower range (Fig. 3f). For instance, maintaining the NaOH concentration and reaction temperature to their center value and then increasing the retention time from 7.6 to 48 h resulted in 78% more soluble compounds, but a further increase of the retention time caused for deterioration of the sCOD yields. The possible reason for the enhancement of sCOD for most levels of the process variables may be attributed to higher hydrolysis of the lignocellulosic materials found in JPC due to the optimum pretreatment conditions [64].
3.3.2 Effect of interactive factors on the degree of solubilization (sCOD)
Although the interaction effects among all variables were not statistically significant (Table 5), the sCOD yields were increased when the value of each process variable increased. More specifically, the amount of sCOD increased when the JPC was pretreated using higher NaOH concentration at the higher temperatures (Fig. 4b). However, a further increment of catalyst concentration (≥ 8%) and incubation temperature (≥ 40 °C) showed a declining trend in sCOD yields. Furthermore, as it is noted from the response surface curve, the effect of the catalyst was more powerful when compared with the temperature at constant retention time (48 h). On a similar vein, the degree of solubilization was increased with increasing of the NaOH concentration from 4 to 8% and retained up to 72 h (Fig. 4d). However, a further increase in NaOH concentration and retention time caused a slight reduction of sCOD yields. Figure 4f shows the amount of sCOD from the JPC increased with the corresponding increment of retention time and incubation temperature at all levels.
The increment in sCOD was associated with the solubilization of cellulose, hemicellulose, and lignin components at the optimum pretreatment conditions. In contrast, the possible reason for lower sCOD yield at severe pretreatment conditions might be due to condensation and precipitation of the solubilized components [65]. Furthermore, the lower sCOD yield could also be associated with instability and formation of complex non-soluble compounds at severe pretreatments [66]. Strong alkali concentration leads to a loss of carbohydrates [67]. The loss of carbohydrates is mainly due to the peeling and hydrolytic reactions, followed by the formation of lower molecular compounds [68]. Moreover, the degradation of these lower molecular compounds at severe pretreatment conditions triggers the loss of organic carbon in the form of carbon dioxide, which could subsequently result in lower sCOD yields. Loss of soluble compounds from cellulose and hemicellulose would increase with increasing NaOH concentration and retention time [35]; this leads to lower sCOD recovery. Furthermore, at a higher temperature, the cross-link between sodium ion and lignin molecule would further decrease the solubilization of lignin during the alkaline pretreatment. As a result, the optimum operational variables for higher sCOD yield were investigated from the model developed using the actual value (Eq. (8)). According to the optimum value suggested by the RSM-CCD, pretreating the JPC with 7.84% NaOH at 37.54 °C for 64.84 h would result in the maximum predicted sCOD value of 163.31 g L−1.
The optimum condition predicted for higher sCOD yields may not be suitable for higher methane production. The possible reasons could be the generation of degraded products like 5-hydroxymethylfurfural (HMF), furfural, and phenolic compounds at severe pretreatment conditions, which all are inhibitor and toxic for microbes involved in the AD process. Furfural and HMF at low levels (≤ 1.4 g L−1) could enhance the biogas production [69]; however, at higher concentrations (≥ 2.0 g L−1), they could disrupt the methanogenic activity [70, 71]. Although alkaline pretreatment is better in carbohydrate preservation as compared with acid pretreatment, the peeling reactions may lead to degradation of polysaccharides with the formation of acids like saccharinic, lactic, formic, and different dihydroxy and dicarboxylic acids [72]. In general, the effect of time was higher when it interacts with a catalyst and temperature, while the impact of the catalyst was powerful when it was combined with temperature. The lower effect of temperature might be attributed to its narrow ranges supplied in the pretreatment process. These temperature ranges were kept intentionally to ensure low energy pretreatment demand, while the lower temperature value (26.6 °C) is the real ambient temperature in Ethiopia where Jatropha is abundantly growing.
3.4 Modeling for biochemical methane potential
In addition to the sCOD, the catalyst concentration (X1), incubation temperature (X2), and retention time (X3) were also selected as main process variables that could affect the methane yield of JPC. The RSM-CCD was employed to investigate the interaction between these process variables and the methane yields obtained after the alkaline pretreatments. Furthermore, RSM-CCD was used to predict the optimum pretreatment conditions for maximum methane production after 61 days of AD. Thus, using Eq. (3) as a generalized model, the actual multiple regression models were developed from the coded and actual experimental data, and the models indicated that a second-order polynomial equation was the best model that describes the relationship between these process variables and the methane yield obtained from JPC (Eqs. (9) and (10)).
where BMP is the biochemical methane potential.
Form Eq. (9), it is clearly shown that the linear effect of NaOH concentration (X1), incubation temperature (X2), and retention time (X3), and the two interaction terms (X1X2 and X1X3) has a positive effect, while all quadratic terms (X12, X22, and X32) and the interaction between X2 and X3 inversely correlated with the methane yield of JPC. Moreover, a multivariate ANOVA was carried out to test the strength of the model that predicted the effect of linear, interactive, and quadratic terms of the X1, X2, and X3 applied during the alkaline pretreatment process. The value for multiple correlation coefficients of R2, adj. R2, and pred. R2 was 0.97, 0.94, and 0.90, respectively, in which the higher R2 value (0.97) indicates that the regression model could not explain only 3% of the total variation in methane yields. The closed correlation between all experimental and model-predicted values indicates the reliable predictive power of the model (Fig. 3a). The effect of linear terms from X1, X2, and X3 and the quadratic terms of the three process variables, i.e., X12, X22, and X32, on methane production of JPC were statistically significant (Table 7).
3.4.1 Effect of independent processing variables on methane yield of JPC
The estimated cumulative methane yield from untreated JPC was 252.41 mL g−1 VS, which is relatively higher than the reported methane yield of sunflower oil cake [60]. Even though the methane yield of pretreated JPC varied across different pretreatment conditions, all values were higher than the methane yield obtained from untreated JPC (Table 8). The increment of cumulative methane yields due to the alkaline pretreatments were ranging from 16.9% (295.10 mL g−1 VS) to 40.2% (353.90 mL g−1 VS). The lower methane yield (295.1 mL g−1 VS) obtained from the alkaline-pretreated JPC was comparable with the literature value of 281 mL g−1 VS obtained after the thermochemical pretreatment [18]. However, in addition to longer digestion time (61 days) requirement, all methane yields were lower than the report by Chandra et al. [73]. The lower methane yield after alkaline pretreatment might be associated with a lack of inherent bacteria that could digest the JPC in the AD process. This effect is strongly supported by Chandra et al. [73] in which maximum methane yield was obtained from JPC within 30 days of digestion time using active bacterial inoculum synthesized from pongamia oil seed cake.
The present study showed that the methane yield of JPC increased by 14% when the concentration of NaOH increased from 2.6 to 6% at constant incubation temperature and retention time of 35 °C and 48 h, respectively (Fig. 3a). However, at the same temperature and retention time, with further increment NaOH concentration to 9.4%, the methane yield of pretreated JPC declined by 1.33%. Similarly, Zheng et al. [43] indicated that adding 6% NaOH into the corn stover achieved 48.5% more biogas yield when compared with the untreated sample. Lower concentration of catalyst during the pretreatment condition leads to a slow rate of lignocellulosic biomass hydrolysis, which subsequently could result in less accessibility to microorganisms during the AD process [60]. Alkaline pretreatment is highly effective in the dissolution of lignin and hemicellulose [62]. However, the hydrolysate at higher NaOH concentration may contain soluble compounds from lignin that may not be metabolized by microbes in the AD process and leads to lower methane yields.
The effect of incubation temperature on methane yield from JPC is shown in Fig. 3c. Soaking the JPC with 6% NaOH and retaining it for 48 h at an incubation temperature of below or above 35 °C resulted in a slight reduction of the methane yields. The methane yield was increased by 13.24% when the applied temperature rose from 26.6 to 35 °C, but it again declined by 8% when the incubation temperature arises from 35 to 43.4 °C. The optimum temperature recorded in this study was in line with Chandra et al. [73], in which the wheat straw pretreated with 4% NaOH (g g−1 TS) at 37 °C for 5 days achieved 112% more methane when compared with the untreated straw.
The effect of retention time on methane yield was investigated at constant NaOH (6%) and incubation temperature (35 °C). The effect of retention time was highly significant at its lower range when compared with the upper values (Fig. 3e). The methane yield was increased by 18.22% when the retention time increased from 7.6 to 48 h. However, when the retention time further increased to 88.4 h, the methane yield increased by only 9.83%. Higher retention time may give a chance of effective hydrolysis of the lignocellulosic component of the press cake and thus leads to fast anaerobic degradation, and then followed by higher methane production [38]. However, retaining the JPC for more extended hours may result in the accumulation of inhibitor compounds such as furfural, HMF, organic acidic, and phenolic compounds, as discussed in Section 3.3.2.
3.4.2 Effect of interactive factors on methane yield of JPC
The methane yield of JPC was increased as a result of increasing the value of pretreatment conditions from their lower to the corresponding center point values (Fig. 4a, c, and e). More specifically, in the lower pretreatment conditions, the methane yield of JPC was increased when both the catalyst concentration and reaction temperature increased (Fig. 4a). However, when the amount of NaOH and incubation temperatures exceed approximately above 8% and 40 °C, respectively, the methane yield started to decline at a slower rate. Similarly, in the lower pretreatment conditions, the methane yield was increased as increasing of NaOH concentration and retention time (Fig. 4c). However, a further increment of NaOH concentration and retention time above 8% and 60 h, respectively, resulted in a decline of methane yields.
The interactive effect between incubation temperature and retention time is also shown in Fig. 4e. The curve from the response surface plot indicates that the methane yield was increased as the incubation temperature rose from 26.4 to 35 °C with the corresponding increment of retention time from 7.6 to 48 h at 6% of NaOH. When the incubation temperature and retention further increased, the methane yields started to decline at a slower rate (Fig. 4e). Comparable with the present study, Sukri et al. [74] indicated that the degradation of carbohydrates due to the higher NaOH concentration (5.25%) and retention time (90 min). In general, maximum methane yields were obtained from JPC at moderate pretreatment conditions. Therefore, the optimum conditions in an alkaline pretreatment were predicted using Eq. (10). The model indicates that pretreating the JPC using 7.32% NaOH at 35.86 °C for 54.05 h would result in maximum methane production of 353.90 mL g−1 VS, which the methane yield was increased by 40.23% as compared with the untreated JPC. The methane yield of JPC obtained at this optimum condition was further evaluated against the literature values, as described in Table 9.
3.5 Energy balance and economic feasibility
Regardless of the costs for biogas plant and pretreatment tank installation, the energy balance and preliminary economic feasibility of the alkaline pretreatment and co-digestion processes were studied. Moreover, the optimum temperature utilized in the alkaline pretreatment was taken as normal temperature for the area from which the sample was brought (Hawassa, Ethiopia). The net increases in methane yields (ΔP) from the alkaline and co-digestion pretreatment processes were estimated to be101.50 and 73.07 L kg−1 VS, respectively. Accordingly, the energy outputs (Eo) calculated from these extra methane yields were 3270.3 and 2354.3 kJ kg−1 VS, respectively. In contrast, the energy input (Ei) required for the alkaline pretreatment was estimated to be 548.8 kJ kg−1 VS, and there was no external energy needed for the co-digestion experiments. Thus, the extra energy obtained after alkaline pretreatment was almost 5.96 times higher as compared with the energy demanded by the NaOH pretreatment.
The BCR analysis was done by considering the unit price of inputs and outputs supplied in the alkaline pretreatment. The cost for one tone of NaOH was estimated to be 412 € t−1 [48], while selling of 1 m3 methane can earn 0.57 € [47]. Thus, pretreating one tone VS of JPC using 7.32% alkaline needed 0.078 t of NaOH, which costs 32.3 €. One tone VS from JPC can produce 101.5 m3 extra methane when compared with the untreated JPC, which prices 57.86 €. Therefore, the BCR obtained by dividing the total methane selling price with NaOH market cost was estimated to be 1.79, which was positive and encourage the application of alkaline pretreatment at a larger scale. The same positive BCR results were obtained by You et al. [35] and Romero-Güiza et al. [47] after alkaline pretreatment of corn stover and wheat straw, respectively. The major challenge of this alkaline pretreatment was the recovery of the added NaOH. Thus, our future research may focus on the recovery of the alkaline chemicals found in hydrolysate using various methods like electro-kinetic cells. The recovery of the chemical could further improve the BCR of the alkaline pretreatment.
4 Conclusions
Alkaline pretreatment and co-digestion processes were identified as effective strategies for enhancing the methane production potential of the JPC and CG. However, the efficiency of the alkaline pretreatment was significantly affected by NaOH concentration, incubation temperature, and retention time. Moreover, the methane yield in the co-digestion process was influenced by FC and CG levels. Both the methane and sCOD yields were declined at severe alkaline pretreatments. The optimum conditions predicted for higher methane and sCOD yields were significantly varied. Variation in optimum conditions defined for maximum methane and sCOD yields could be used as an indicator of process inhibition, probably due to the accumulation of degraded products at severe alkaline pretreatment. The positive energy balance and higher BCR inspire the application of the alkaline pretreatment at a larger scale for enhancing the methane yield of JPC. To reduce the downstream processing cost and effective utilization of the catalyst, the future research work in this area should focus on the recovery of NaOH from the hydrolysate using different techniques.
Abbreviations
- adj. R 2 :
-
Adjacent R square
- APHA:
-
American Public Health Association
- NH3 :
-
Ammonia
- NH4OH:
-
Ammonium hydroxide
- AD:
-
Anaerobic digestion
- ANOVA:
-
Analysis of variance
- BCR:
-
Benefit-cost ratio
- BMP:
-
Biochemical methane potential
- Ca(OH)2 :
-
Calcium hydroxide
- CO2 :
-
Carbon dioxide
- C/N :
-
Carbon-to-nitrogen ratio
- CCD:
-
Central composite design
- CG:
-
Crude glycerol
- °C:
-
Degree Celsius
- E i :
-
Energy input
- E o :
-
Energy output
- EPA:
-
Environmental protection agency
- ɳ :
-
Eta (Greek letter)
- FC:
-
Feedstock concentration
- GC :
-
Gas chromatography
- g:
-
Gram
- H2O2 :
-
Hydrogen peroxide
- ISR:
-
Inoculum-to-substrate ratio
- JPC:
-
Jatropha press cake
- K:
-
Kelvin
- kg:
-
Kilogram
- kJ:
-
Kilojoule
- kPa:
-
Kilopascal
- L:
-
Liter
- MRV:
-
Measurement, reporting, and verification
- MJ:
-
Mega joule
- m3 :
-
Meter cubic
- CH4 :
-
Methane
- MC:
-
Moisture content
- R 2 :
-
Multiple R square
- NIBIO:
-
Norwegian Institute of Bioecnomy Research
- pred. R 2 :
-
Predicted R square
- RSM:
-
Response surface methodology
- rpm:
-
Revolution per minute
- NaOH:
-
Sodium hydroxide
- sCOD:
-
Soluble chemical oxygen demand
- TCD:
-
Thermal conductivity detector
- TS:
-
Total solid
- VFA:
-
Volatile fatty acid
- VS:
-
Volatile solid
- wt:
-
Weight
- ξ :
-
Xi (Greek letter)
- HMF:
-
5-hydroxymethylfurfural
References
IEA (2019) International Energy Outlook 2019 with projections to 2050. U.S. Energy Information Administration; Office of Energy Analysis; U.S. Department of Energy, Washington, DC 20585, USA
Schwingel AW, Orrico AC, de Lucas JJ, Junior MA, Borquis RR, Fava F (2019) Laying hen manure in anaerobic co-digestion with glycerin containing different glycerol and impurity levels. J Clean Prod 215:1437–1444
Bazilian M, Sagar A, Detchon R, Yumkella K (2010) More heat and light. Energy Policy 38:5409–5412
Martha N, Riera O (2014) Biodiesel value chain and access to energy in Ethiopia: policies and business prospects. Renew Sust Energ Rev 39:975–985. https://doi.org/10.1016/j.rser.2014.07.152
Mondal MAH, Bryan E, Ringler C, Mekonnen D, Rosegrant M (2018) Ethiopian energy status and demand scenarios: prospects to improve energy efficiency and mitigate GHG emissions. Energy 149:161–172. https://doi.org/10.1016/j.energy.2018.02.067
Tesfaye K (2011) Biodiesel production from Wado seed oil. PhD Dissertation Addis Ababa, Addis Ababa, Ethiopia
NBE (2019) Anual Report. National Bank of Ethiopia (NBE), Addis Ababa, Ethiopa
Commission NP (2016) Growth and transformation plan II (GTP II) (2015/16–2019/20), vol 1. National Planning Commission, Addis Ababa
Portner B, Ehrensperger A, Nezir Z, Breu T, Hurni H (2014) Biofuels for a greener economy? Insights from Jatropha production in northeastern Ethiopia. Sustainability 6(9):6188–6202. https://doi.org/10.3390/su6096188
Jonas M, Ketlogetswe C, Gandure J (2020) Variation of Jatropha curcas seed oil content and fatty acid composition with fruit maturity stage. Heliyon 6(1):e03285. https://doi.org/10.1016/j.heliyon.2020.e03285
Supamathanon N, Wittayakun J, Prayoonpokarach S (2011) Properties of Jatropha seed oil from northeastern Thailand and its transesterification catalyzed by potassium supported on NaY zeolite. J Ind Eng Chem 17(2):182–185. https://doi.org/10.1016/j.jiec.2011.02.004
Zhang X, Yang Z, Liang J, Tang L, Chen F (2016) Detoxification of Jatropha curcas seed cake in solid-state fermentation of newly isolated endophytic strain and nutrition assessment for its potential utilizations. Int Biodeterior Biodegradation 109:202–210. https://doi.org/10.1016/j.ibiod.2016.02.001
Zhang X, Yan S, Tyagi RD, Surampalli RY, Valero JR (2016) Energy balance of biofuel production from biological conversion of crude glycerol. J Environ Manag 170:169–176. https://doi.org/10.1016/j.jenvman.2015.09.031
Sushma (2014) Analysis of oil content of Jatropha curcas seeds under storage condition. J Environ Biol 35:571–575
Abou-Arab AA, Mahmoud MH, Ahmed DMM, Abu-Salem FM (2019) Comparative study between chemical, physical and enzymatic methods for Jatropha curcas kernel meal phorbol ester detoxification. Heliyon 5(5):e01689. https://doi.org/10.1016/j.heliyon.2019.e01689
Raheman H, Mondal S (2012) Biogas production potential of jatropha seed cake. Biomass Bioenergy 37:25–30. https://doi.org/10.1016/j.biombioe.2011.12.042
Adekunle AS, Ibitoye SE, Omoniyi PO (2019) Production and testing of biogas using cow dung, jatropha and iron filins. JB&B 2(4):143–148. https://doi.org/10.12162/jbb.v4i3.002
Jablonski SJ, Kulazynski M, Sikora I, Lukaszewicz M (2017) The influence of different pretreatment methods on biogas production from Jatropha curcas oil cake. J Environ Manag 203(Pt 2):714–719. https://doi.org/10.1016/j.jenvman.2016.06.001
Liang Y, Siddaramu T, Yesuf J, Sarkany N (2010) Fermentable sugar release from Jatropha seed cakes following lime pretreatment and enzymatic hydrolysis. Bioresour Technol 101:6417–6424
Kumar G, Sen B, Lin CY (2013) Pretreatment and hydrolysis methods for recovery of fermentable sugars from de-oiled Jatropha waste. Bioresour Technol 145:275–279. https://doi.org/10.1016/j.biortech.2013.02.080
Abraham A, Mathew AK, Park H, Choi O, Sindhu R, Parameswaran B, Pandey A, Park JH, Sang BI (2020) Pretreatment strategies for enhanced biogas production from lignocellulosic biomass. Bioresour Technol 301:122725. https://doi.org/10.1016/j.biortech.2019.122725
Millati R, Wikandari R, Ariyanto T, Putri RU, Taherzadeh MJ (2020) Pretreatment technologies for anaerobic digestion of lignocelluloses and toxic feedstocks. Bioresour Technol 304:122998. https://doi.org/10.1016/j.biortech.2020.122998
Tabatabaei M, Aghbashlo M, Valijanian E, Kazemi Shariat Panahi H, Nizami A-S, Ghanavati H, Sulaiman A, Mirmohamadsadeghi S, Karimi K (2020) A comprehensive review on recent biological innovations to improve biogas production, part 2: mainstream and downstream strategies. Renew Energy 146:1392–1407. https://doi.org/10.1016/j.renene.2019.07.047
Amin FR, Khalid H, Zhang H, Rahman SU, Zhang R, Liu G, Chen C (2017) Pretreatment methods of lignocellulosic biomass for anaerobic digestion. AMB Express 7(1):72. https://doi.org/10.1186/s13568-017-0375-4
Haider MR, Zeshan YS, Malik RN, Visvanathan C (2015) Effect of mixing ratio of food waste and rice husk co-digestion and substrate to inoculum ratio on biogas production. Bioresour Technol 190:451–457. https://doi.org/10.1016/j.biortech.2015.02.105
Kamusoko R, Jingura RM, Parawira W, Sanyika WT (2019) Comparison of pretreatment methods that enhance biomethane production from crop residues—a systematic review. Biofuel Res J 6(4):1080–1089. https://doi.org/10.18331/brj2019.6.4.4
Estevez MM, Linjordet R, Morken J (2012) Effects of steam explosion and co-digestion in the methane production from Salix by mesophilic batch assays. Bioresour Technol 104:749–756. https://doi.org/10.1016/j.biortech.2011.11.017
Horn SJ, Estevez MM, Nielsen HK, Linjordet R, Eijsink VG (2011) Biogas production and saccharification of Salix pretreated at different steam explosion conditions. Bioresour Technol 102(17):7932–7936. https://doi.org/10.1016/j.biortech.2011.06.042
Cheng YS, Zheng Y, Yu CW, Dooley TM, Jenkins BM, VanderGheynst JS (2010) Evaluation of high solids alkaline pretreatment of rice straw. Appl Biochem Biotechnol 162(6):1768–1784. https://doi.org/10.1007/s12010-010-8958-4
Iram A, Cekmecelioglu D, Demirci A (2019) Optimization of dilute sulfuric acid, aqueous ammonia, and steam explosion as the pretreatments steps for distillers’ dried grains with solubles as a potential fermentation feedstock. Bioresour Technol 282:475–481. https://doi.org/10.1016/j.biortech.2019.03.009
Tabatabaei M, Aghbashlo M, Valijanian E, Kazemi Shariat Panahi H, Nizami A-S, Ghanavati H, Sulaiman A, Mirmohamadsadeghi S, Karimi K (2020) A comprehensive review on recent biological innovations to improve biogas production, part 1: upstream strategies. Renew Energy 146:1204–1220. https://doi.org/10.1016/j.renene.2019.07.037
Pang YZ, Liu YP, Li XJ, Wang KS, Yuan HR (2008) Improving biodegradability and biogas production of corn Stover through sodium hydroxide solid state pretreatment. Energy Fuel 22:2761–2766
B-l D, Guo X-j, D-h Y, Xu J-m (2017) Comparison of different pretreatments of rice straw substrate to improve biogas production. Waste Biomass Valor 9(9):1503–1512. https://doi.org/10.1007/s12649-017-9950-9
Mustafa AM, Li H, Radwan AA, Sheng K, Chen X (2018) Effect of hydrothermal and Ca(OH)2 pretreatments on anaerobic digestion of sugarcane bagasse for biogas production. Bioresour Technol 259:54–60. https://doi.org/10.1016/j.biortech.2018.03.028
You Z, Pan S-Y, Sun N, Kim H, Chiang P-C (2019) Enhanced corn-stover fermentation for biogas production by NaOH pretreatment with CaO additive and ultrasound. J Clean Prod:238. https://doi.org/10.1016/j.jclepro.2019.117813
Yuan Z, Wei W, Wen Y, Wang R (2019) Comparison of alkaline and acid-catalyzed steam pretreatments for ethanol production from tobacco stalk. Ind Crop Prod 142. https://doi.org/10.1016/j.indcrop.2019.111864
Ozbayram EG, Ince O, Ince B, Harms H, Kleinsteuber S (2018) Comparison of rumen and manure microbiomes and implications for the inoculation of anaerobic digesters. Microorganisms 6(1). https://doi.org/10.3390/microorganisms6010015
Zhu J, Wan C, Li Y (2010) Enhanced solid-state anaerobic digestion of corn stover by alkaline pretreatment. Bioresour Technol 101(19):7523–7528. https://doi.org/10.1016/j.biortech.2010.04.060
Sambusiti C, Monlau F, Ficara E, Carrère H, Malpei F (2013) A comparison of different pre-treatments to increase methane production from two agricultural substrates. Appl Energy 104:62–70. https://doi.org/10.1016/j.apenergy.2012.10.060
Li L, Chen C, Zhang R, He Y, Wang W, Liu G (2015) Pretreatment of corn stover for methane production with the combination of potassium hydroxide and calcium hydroxide. Energy Fuel 29(9):5841–5846. https://doi.org/10.1021/acs.energyfuels.5b01170
Pellera FM, Santori S, Pomi R, Polettini A, Gidarakos E (2016) Effect of alkaline pretreatment on anaerobic digestion of olive mill solid waste. Waste Manag 58:160–168. https://doi.org/10.1016/j.wasman.2016.08.008
Svensson K, Kjørlaug O, Horn SJ, Agger JW (2017) Comparison of approaches for organic matter determination in relation to expression of bio-methane potentials. Biomass Bioenergy 100:31–38. https://doi.org/10.1016/j.biombioe.2017.03.005
Zheng M, Li X, Li L, Yang X, He Y (2009) Enhancing anaerobic biogasification of corn Stover through wet state NaOH pretreatment. Bioresour Technol 100(21):5140–5145. https://doi.org/10.1016/j.biortech.2009.05.045
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington, DC
Bauer A, Lizasoain J, Theuretzbacher F, Agger JW, Rincon M, Menardo S, Saylor MK, Enguidanos R, Nielsen PJ, Potthast A, Zweckmair T, Gronauer A, Horn SJ (2014) Steam explosion pretreatment for enhancing biogas production of late harvested hay. Bioresour Technol 166:403–410. https://doi.org/10.1016/j.biortech.2014.05.025
Ferreira SF, Buller LS, Berni MD, Bajay SV, Forster-Carneiro T (2019) An integrated approach to explore UASB reactors for energy recycling in pulp and paper industry: a case study in Brazil. Biofuel Res J 6(3):1039–1045. https://doi.org/10.18331/brj2019.6.3.4
Romero-Guiza MS, Wahid R, Hernandez V, Moller H, Fernandez B (2017) Improvement of wheat straw anaerobic digestion through alkali pre-treatment: carbohydrates bioavailability evaluation and economic feasibility. Sci Total Environ 595:651–659. https://doi.org/10.1016/j.scitotenv.2017.04.006
Dahunsi SO, Oranusi S, Efeovbokhan VE (2017) Pretreatment optimization, process control, mass and energy balances and economics of anaerobic co-digestion of Arachis hypogaea (Peanut) hull and poultry manure. Bioresour Technol 241:454–464. https://doi.org/10.1016/j.biortech.2017.05.152
Ganidi N, Tyrrel S, Cartmell E (2011) The effect of organic loading rate on foam initiation during mesophilic anaerobic digestion of municipal wastewater sludge. Bioresour Technol 102(12):6637–6643. https://doi.org/10.1016/j.biortech.2011.03.057
Kougias PG, Boe K, Angelidaki I (2013) Effect of organic loading rate and feedstock composition on foaming in manure-based biogas reactors. Bioresour Technol 144:1–7. https://doi.org/10.1016/j.biortech.2013.06.028
Duan N, Zhang D, Lin C, Zhang Y, Zhao L, Liu H, Liu Z (2019) Effect of organic loading rate on anaerobic digestion of pig manure: methane production, mass flow, reactor scale and heating scenarios. J Environ Manag 231:646–652
Chen Y, Cheng JJ, Creamer KS (2008) Inhibition of anaerobic digestion process: a review. Bioresour Technol 99(10):4044–4064
Astals S, Nolla-Ardevol V, Mata-Alvarez J (2013) Thermophilic co-digestion of pig waste and crude glycerol: process performance and digestate stability. J Biotechnol 166:97–104
Oliveira JV, Alves MM, Costa JC (2015) Optimization of biogas production from Sargassum sp. using a design of experiments to assess the co-digestion with glycerol and waste frying oil. Bioresour Technol 175:480–485. https://doi.org/10.1016/j.biortech.2014.10.121
Veroneze ML, Schwantes D, Gonçalves AC, Richart A, Manfrin J, da Paz SA, Schuba TB (2019) Production of biogas and biofertilizer using anaerobic reactors with swine manure and glycerin doses. J Clean Prod 213:176–184. https://doi.org/10.1016/j.jclepro.2018.12.181
Ganidi N, Tyrrel S, Cartmell E (2009) Anaerobic digestion foaming causes—a review. Bioresour Technol 100(23):5546–5554. https://doi.org/10.1016/j.biortech.2009.06.024
Kougias PG, Boe K, Einarsdottir ES, Angelidaki I (2015) Counteracting foaming caused by lipids or proteins in biogas reactors using rapeseed oil or oleic acid as antifoaming agents. Water Res 79:119–127. https://doi.org/10.1016/j.watres.2015.04.034
Haitl M, Vítez T, Koutný T, Kukla R, Losak T, Gadus J (2012) Use of G-phase for biogas production. Acta Univ Agric Silvic Mendelianae Brun 60(6):89–95
Subroto E, Manurung R, Heeres HJ, Broekhuis AA (2015) Optimization of mechanical oil extraction from Jatropha curcas L. kernel using response surface method. Ind Crop Prod 63:294–302. https://doi.org/10.1016/j.indcrop.2014.08.050
Monlau F, Latrille E, Da Costa AC, Steyer J-P, Carrère H (2013) Enhancement of methane production from sunflower oil cakes by dilute acid pretreatment. Appl Energy 102:1105–1113. https://doi.org/10.1016/j.apenergy.2012.06.042
Perendeci NA, Gokgol S, Orhon D (2018) Impact of alkaline H(2)O(2) pretreatment on methane generation potential of greenhouse crop waste under anaerobic conditions. Molecules 23(7). https://doi.org/10.3390/molecules23071794
Kim JS, Lee YY, Kim TH (2016) A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour Technol 199:42–48. https://doi.org/10.1016/j.biortech.2015.08.085
Modenbach A (2013) Sodium hydroxide pretreatment of corn stover and subsequent enzymatic hydrolysis: an investigation of yields, kinetic modeling and glucose recovery theses and dissertations, University of Kentucky, Biosystems and Agricultural Engineering
Zhang Q, Tang L, Zhang J, Mao Z, Jiang L (2011) Optimization of thermal-dilute sulfuric acid pretreatment for enhancement of methane production from cassava residues. Bioresour Technol 102(4):3958–3965. https://doi.org/10.1016/j.biortech.2010.12.031
Gonzales M, Assadi M, Ouki S (2003) Effect of sludge composition on the degree of stabilisation and gas production during Mesophilic anaerobic digestion (MAD). Paper presented at the the 8th European biosolids and organic residuals conference, Wakefield, UK, NoV-24-26
Lopez Torres M, Espinosa Llorens Mdel C (2008) Effect of alkaline pretreatment on anaerobic digestion of solid wastes. Waste Manag 28(11):2229–2234. https://doi.org/10.1016/j.wasman.2007.10.006
Hendriks AT, Zeeman G (2009) Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol 100(1):10–18. https://doi.org/10.1016/j.biortech.2008.05.027
Fengel D, Wegener G (1984) Wood: chemistry, ultrastructure, reactions. Walter de Gruyter, Berlin
Boopathy R (2009) Anaerobic biotransformation of furfural to furfuryl alcohol by a methanogenic archaebacterium. Int Biodeterior Biodegradation 63(8):1070–1072. https://doi.org/10.1016/j.ibiod.2009.09.011
Ghasimi DSM, Aboudi K, de Kreuk M, Zandvoort MH, van Lier JB (2016) Impact of lignocellulosic-waste intermediates on hydrolysis and methanogenesis under thermophilic and mesophilic conditions. Chem Eng J 295:181–191. https://doi.org/10.1016/j.cej.2016.03.045
Phuttaroa SC, Surendrab KC, Boonsawang P, Chaiprapata S, Khanalb S (2019) Anaerobic digestion of hydrothermally-pretreated lignocellulosic biomass: influence of pretreatment temperatures, inhibitors and soluble organics on methane yield. Bioresour Technol 284:128–138. https://doi.org/10.1016/j.biortech.2019.03.114
Jonsson LJ, Martin C (2016) Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Bioresour Technol 199:103–112. https://doi.org/10.1016/j.biortech.2015.10.009
Chandra R, Takeuchi H, Hasegawa T, Kumar R (2012) Improving biodegradability and biogas production of wheat straw substrates using sodium hydroxide and hydrothermal pretreatments. Energy 43(1):273–282. https://doi.org/10.1016/j.energy.2012.04.029
Sukri SSM, Rahman RAA, Illias RM, Yaakob H (2014) Optimization of alkaline pretreatment conditions of oil palm fronds in improving the lignocelluloses contents for reducing sugar production. Roman Biotechnol Lett 19:9006–9018
Khor WC, Rabaey K, Vervaeren H (2015) Low temperature calcium hydroxide treatment enhances anaerobic methane production from (extruded) biomass. Bioresour Technol 176:181–188. https://doi.org/10.1016/j.biortech.2014.11.037
Li Y, Merrettig-Bruns U, Strauch S, Kabasci S, Chen H (2015) Optimization of ammonia pretreatment of wheat straw for biogas production. J Chem Technol Biotechnol 90(1):130–138. https://doi.org/10.1002/jctb.4297
Acknowledgments
Our special thanks go to Roald Aasen and Hege Bergheim for their unreserved support while doing the laboratory analysis. Furthermore, we would like to thank all staff members at the Norwegian Institute of Bioeconomy Research (NIBIO) who were working in the biogas laboratory for their knowledge sharing and technical support.
Funding
Open Access funding provided by Norwegian University of Life Sciences. The study is financially supported by the National Capacity Building on Measurement, Reporting, and Verification (MRV) Project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ewunie, G.A., Morken, J. & Yigezu, Z.D. Alkaline and co-digestion pretreatments: process optimization for enhancing the methane yield of Jatropha press cake. Biomass Conv. Bioref. 11, 971–988 (2021). https://doi.org/10.1007/s13399-020-00732-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00732-y