Abstract
In this study, we report the synthesis and biological evaluation of a variety of α- and β-hydroxy substituted amino acid derivatives as potential amino acid subunits in inhibitors of GABA uptake transporters (GATs). In order to ensure that the test compounds adopt a binding pose similar to that presumed for related larger GAT inhibitors, lipophilic residues were introduced either at the amino nitrogen atom or at the alcohol function. Several of the synthesized compounds were found to exhibit similar inhibitory activity at the GAT subtypes mGAT2, mGAT3, and mGAT4, respectively, as compared with the reference N-butylnipecotic acid. Hence, these compounds might serve as starting point for future developments of more complex GAT inhibitors.
Similar content being viewed by others
Introduction
Gamma-aminobutyric acid (GABA) is the most abundant inhibitory neurotransmitter in the mammalian central nervous system (CNS) (Bowery and Smart 2006), with up to 40% of synapses estimated to be GABAergic (Meldrum and Chapman 1999). Deficient GABAergic neurotransmission is assumed to play a decisive role in the pathogenesis of several severe neurological diseases, including Alzheimer’s disease (Lanctôt et al. 2004), depression (Kalueff and Nutt 2007), epilepsy (Treimann 2001), and neuropathic pain (Daemen et al. 2008). A promising therapeutical approach for the treatment of these diseases exists in the inhibition of the transport molecules responsible for the removal of GABA from the synaptic cleft, resulting in the prolongation of the effect exerted by the available GABA (Krogsgaard-Larsen et al. 1991). Belonging to the solute carrier 6 (SLC6) transporter gene family, these membrane-bound proteins termed GABA transporters (GATs) use the co-transport of sodium ions for the translocation of the substrate against the chemical gradient (Kristensen et al. 2011). The nomenclature of the four GAT subtypes depends on the species the transporters are cloned from. When originating from mouse brain cells, the GAT subtypes are termed mGAT1–4. For all other species, including humans, an alternate nomenclature is used which has also been adopted by the Human Genome Organisation, denoting the transporters GAT1 (=mGAT1), BGT1 (=mGAT2), GAT2 (=mGAT3), and GAT3 (=mGAT4) (Madsen et al. 2009). Hereafter the nomenclature referring to the murine transporters will be applied as the biological test system used in our group is based on these. mGAT1 and mGAT4 are the most abundant GATs in the mammalian CNS, with the former being predominantly located on presynaptic neuronal membranes mediating the neuronal GABA uptake, whereas mGAT4, which is mainly expressed on glia cells, is responsible for the glial GABA uptake (Minelli et al. 1996; Jin et al. 2011). The other two GAT subtypes, mGAT2 and mGAT3, are primarily located in the periphery, with the highest densities being found in liver and kidneys, and hence are thought to not play any significant role in the termination of GABAergic signalling in the CNS (Zhou et al. 2012).
The first parent compound selectively targeting GATs was 4,5,6,7-tetrahydroixoxazolo[4,5-c]pyridin-3-ol (THPO, 1, Table 1, entry 1) (White et al. 2002), which is derived from muscimol, an alkaloid isolated from fly agaric (Amanita muscaria) that can be considered an bioisostere of GABA (Corvey et al. 1994). Replacement of the isoxazol-3-ol partial structure of THPO with a carboxylic acid function led to the more potent GAT inhibitors guvacine (2, Table 1, entry 2) and nipecotic acid [(R)-3,(S)-3 Table 1, entry 3–4] (Krogsgaard-Larsen et al. 2000). However, these cyclic GABA analogues still lacked inhibitory potency and subtype selectivity (Table 1, entry 1–4). Moreover, due to being present in the zwitterionic state under physiological conditions, guvacine (2) and nipecotic acid [(R)-3,(S)-3] are hardly able to cross the blood brain barrier (BBB), which strongly limits any potential therapeutic application (Seth et al. 2018).
When bulky, lipophilic residues were introduced at the nitrogen atom, this led to compounds with not only increased lipophilicity and hence improved BBB penetration, but also with significantly higher inhibitory potency. In this context, compounds possessing a diaryl methyl or a biaryl unit as lipophilic domain which is connected to the nipecotic acid partial structure via a flexible linker of 3–5 atoms proved to be highly potent and selective mGAT1 inhibitors, with the respective pIC50 values rising from ~5 to almost 7 as compared with unsubstituted (R)-nipecotic acid. Among these compounds, Tiagabine (Gabatril®, 4, Table 1, entry 5) stands out for being the only GAT inhibitor that has been approved for clinical use (Nielsen et al. 1991).
In a similar way the introduction of a lipophilic residue consisting of a triarylmethyl group, which is linked to the nipecotic acid subunit via a spacer of three atoms, furnishes mGAT4 selective inhibitors, with (S)-SNAP-5114 constituting the prototypic representative of this group (5, Table 1, entry 6) (Dhar et al. 1994). Remarkably, the (S)-isomer 5 exhibits higher inhibitory potency than the respective (R)-isomer, running contrary to what is observed for the unsubstituted nipecotic acid [(R)-3, (S)-3] as well as for mGAT1 inhibitors such as Tiagabine (4).
Interestingly, small amino acids including β-alanine (6, Table 2, entry 1), isoserine (7a, Table 2, entry 2), 2,3-diaminopropionic acid (8, Table 2, entry 3) and (Z)-4-aminobut-2-enoic acid (9, Table 2, entry 4) also show moderate biological activity at mGAT3 and mGAT4 that is comparable to that exerted by (R)-nipecotic acid [(R)-3, Table 1, entry 3], while being distinctly less potent inhibitors of mGAT1 (Kragler et al. 2005). Thus, substitution of the nipecotic acid partial structure in the lead compound (S)-SNAP-5114 (5) with a (S)-2-hydroxy-2-[(R)-pyrrolidin-2-yl]acetic acid unit, which can be understood as rigidized derivative of rac-isoserine (7a), led to compound 10, which displays significantly improved subtype selectivity in favour of mGAT4. Unfortunately, this structure variation is also accompanied by a moderate decrease of inhibitory potency (pIC50 = 5.18 ± 0.05, Table 2, entry 5) as compared with the parent compound 5 (pIC50 = 5.71 ± 0.20, Table 1, entry 6) (Steffan et al. 2015).
For the development of mGAT4 inhibitors with increased subtype selectivity, this study hence aims to identify further cyclic and acyclic 2- and 3-hydroxyamino acids as possible alternatives to the nipecotic acid partial structure present in the scaffold of important mGAT4 inhibitors such as 5. In this context, the hydroxyl function plays a crucial role as it may enable additional interactions with the target, e.g., by establishing hydrogen bridges, hence increasing binding enthalpy. Proceeding from the basic structure of isoserine 7a, it was intended to implement several structural modifications, including elongation of the carbon chain by insertion of methylene groups at various positions, and the rigidization of the molecule by integrating the amino acid backbone into larger heterocycles. Accordingly, a set of compounds featuring both variations and thus deviating from the original isoserine (7a) structure should be synthesized and biologically evaluated (Scheme 1).
Despite the stereochemistry of amino acids such as nipecotic acid [(R)-3, (S)-3] being known to represent an important factor when it comes to the biological activity of mGAT4 inhibitors, we opted for the synthesis of the target compounds in racemic form as this provides information about the biological activity of both enantiomers.
According to the results of molecular modelling experiments previously performed by us for mGAT1 (Wein et al. 2016), small inhibitors such as nipecotic acid adapt a binding pose at which the amino nitrogen atom is facing towards the intracellular space. However, if bulky, lipophilic moieties are introduced at the nitrogen atom, as it is the case with Tiagabine (4, Table 1, entry 5), the binding pose is altered in a way that the nitrogen atom is orientated towards the extracellular site, with the lipophilic side chain looming into the extracellular vestibule, although the position of the carboxylic acid function remains largely unchanged (Scheme 2). Using the example of a N-butyl residue, Wein et al. demonstrated that even the presence of small N-substituents is sufficient to cause this change of the binding mode. Although no reliable in silico models exists so far for the other GAT subtypes, it seems highly likely that these findings also apply to mGAT2-4. Thus, following this assumption, all parent compounds included in this study were also evaluated for their inhibitory potential as N-butyl derivatives in order to ensure that the orientation of the parent compounds in the binding pocket corresponds to the orientation they would have as part of larger molecules comprising a lipophilic domain.
In addition, unsubstituted α- and β-hydroxyamino acids exhibiting higher biological activity than their corresponding N-butyl derivatives should in addition be substituted at the alcohol function. In detail, the alcohol function should be provided with a 4,4′,4″-trimethoxytrityloxyethyl residue [(4-MeO-C6H4)3COCH2CH2], which is a characteristic structural motif of mGAT4 inhibitors such as 5 and 10. According to the model pointed out above, this might allow the amino acid substructure to stay in the position with the nitrogen atom facing towards the intracellular space, which would evidently be more favourable than the antagonal orientation found in the N-substituted amino acids. At the same time, the newly introduced lipophilic domain might be accommodated in the vestibule, which is known to contribute in general significantly to inhibitory potency and selectivity of GAT inhibitors (compare Table 1, entries 3 and 4 with 6).
Materials and methods
Chemistry
Moisture-sensitive reactions were carried out in oven-dried glassware under inert gas atmosphere. Commercially available starting materials were used without further purification. Tetrahydrofuran (THF) was freshly distilled from sodium benzophenone ketyl. All other solvents were distilled prior to use. Microwave reactions were carried out with Biotage Initiator™. Flash column chromatography was performed on Merck silica gel 60 (mesh 0.040–0.063 mm) as stationary phase; thin-layer chromatography (TLC) was carried out on Merck silica gel 60 F254 sheets. Preparative MPLC was performed using a Buechi instrument (C-605 binary pump system, C-630 UV detector at 254 nm and C-660 fraction collector) and a Sepacore B-685 (26 V, 230 mm) glass column equipped with YMC Gel Triart Prep C18-S (12 nm, 5–20 µm). 1H and 13C NMR spectra were, unless stated otherwise, recorded at rt with JNMR-GX (JEOL 400 or 500 MHz) or Bruker BioSpin Avance III HD (400 or 500 MHz) and integrated with the NMR software MestReNova. Deuterated solvents for NMR, which included CD2Cl2, CDCl3, MeOD, D2O, and 1,1,2,2-tetrachloroethane-d2, were purchased from Eurisotop, Saint-Aubin, France. NaOD was purchased from Sigma-Aldrich Chemie GmbH, Munich, Germany. IR samples were measured as KBr pellets or film with Perkin-Elmer FT-IR 1600. HRMS data were obtained with JMS-GCmate II (EI, Jeol) or Thermo Finnigan LTQ FT Ultra (ESI, Thermo Finnigan).
General procedure for the synthesis of the β-hydroxyamino acid esters (GP1)
A solution of lithium-HMDS in MTBE (0.97 M) was cooled to −78 °C and anhydrous ethyl acetate (EtOAc, 1.0 eq) was added dropwise. After complete addition the mixture was stirred for 25 min and a solution of ketone (1.0 eq) in dry THF was added. The reaction mixture was allowed to slowly warm up to −10 °C, quenched with brine, diluted with H2O and extracted thrice with CH2Cl2. The combined CH2Cl2 phases were dried over sodium sulfate (Na2SO4) and concentrated in vacuum to yield the crude product.
General procedure for the deprotection and N-butylation of the amino acid amides and amino acid esters (GP2)
A mixture of the benzyl or benzhydryl-protected amino acid derivative, palladium on charcoal (10% Pd, 0.1 eq) and butyraldehyde (2.5 eq) in EtOH was stirred vigorously under 15 bar hydrogen pressure for 16 h, filtered over Cealite® and reduced in vacuum.
General procedure for the hydrolysis of the amides and esters (GP3)
A mixture of carboxamide or ester and barium hydroxide octahydrate (2.0–2.4 eq) was stirred under reflux conditions (carboxamides) or at rt (esters) in EtOH/H2O 1:1 for the appropriate time. Carbon dioxide was passed through until no further precipitate formed. The suspension was filtered over a cotton wool pad and a syringe filter (Perfect-Flow®, WICOM Germany GmbH, PTFE, 0.2 µM) and reduced in vacuum. The residue was solved in distilled water (2.0 ml) and lyophilized.
General procedure for the N-butylation of the acyclic amino acids (GP4)
The amino acid (1.0 eq) and KOH (2.0 eq) were suspended in EtOH and H2O was added until the reaction mixture became homogeneous. 1-Bromobutane (0.9 eq) was added dropwise. After stirring at rt for 16 h the reaction mixture was reduced in vacuum. The crude compound was purified by MPLC (eluent: MeOH/H2O 1:9).
General procedure for the protection of the acyclic amino acids I (GP5a)
A solid, well-grounded mixture of the amino acid (1.0 eq) and phtalic anhydride (1.0 eq) was heated to 140 °C, resulting in a colourless melting. After 30 min, the reaction mixture was cooled to rt and re-dissolved in EtOAc (300 ml). The solution was washed with 1 M sodium hydrogen sulfate solution (100 ml), water (3 × 100 ml), and brine (100 ml). The organic layer was dried over Na2SO4, filtered, and concentrated in vacuum. The resulting residue was solved in anhydrous MeOH (250 ml) and 2 M HCl in Et2O (250 ml) was added. The mixture was stirred at rt until TLC indicated complete consumption of the educt and reduced in vacuum. The crude compound was purified by flash column chromatography on silica (eluent: CH2Cl2/EtOAc 9:1).
General procedure for the protection of the acyclic amino acids II (GP5b)
A solid, well-grounded mixture of the amino acid (1.0 eq) and phtalic anhydride (1.0 eq) was heated to 140 °C, resulting in a colourless melting. After 30 min, the reaction mixture was cooled to rt, solved in anhydrous MeOH (250 ml) and 2M HCl in Et2O (250 ml) was added. The mixture was stirred at rt until TLC indicated complete consumption of the educt and reduced in vacuum. The crude compound was purified by flash column chromatography on silica (eluent: CH2Cl2/EtOAc 9:1).
General procedure for the formation of the ether function (GP6)
tert-Butyl(2-iodoethoxy) diphenylsilane (1.4 eq) and Ag2CO3 (4.0 eq) were added to a suspension of the amino acid derivative (1.0 eq) in toluene (10.0 ml). The reaction mixture was stirred in a pressure tube at 120 °C until TLC indicated complete consumption of the amino acid derivative, cooled to rt, filtered through a paper filter and reduced in vacuum. The residue was purified by flash column chromatography on silica (eluent: pentane/Et2O 7:3).
General procedure for cleavage of the TBDPS protecting group (GP7)
The TBDPS-protected compound (1.0 eq) was solved in THF/pyridine 9:1 (v/v) in a polypropylene tube. A 70% solution of HF-pyridine (5.0 eq) was added dropwise at 0 °C. The suspension was stirred at rt and the progress of the reaction was monitored by TLC. After complete consumption of the educt phosphate buffer (pH = 6.0, 1.0 M, 100 ml) was added and the mixture was extracted with ethyl acetate (100 ml). The organic phase was washed with water (100 ml) and brine (100 ml), dried over MgSO4 and reduced in vacuum. The crude product was purified by flash column chromatography on silica (eluent: CH2Cl2/EtOAc 65:35).
General procedure for the coupling of the alcohol with 4,4′,4″-trimethoxytrithyl chloride (GP8)
The alcohol (1.0 eq) was solved in pyridine. Dimethylformamide (DMF, 1 drop) and 4,4′,4″-trimethoxytrithyl chloride (1.8 eq.) were added. The mixture was stirred at 55 °C for 16 h and reduced in vacuum. The crude product was purified by flash chromatography on silica (eluent Et2O/pentane: 6:4).
General procedure for the deprotection of the acyclic amino acid derivatives I (GP9a)
12 M NaOH (2.0 eq) was added to a solution of the protected compound (1.0 eq) in MeOH (15.0 ml). After stirring for 16 h at rt, 1,2-diaminoethane (7.0 eq) was added and the mixture was heated in a microwave for 16 h at 140 °C. Finally, the reaction mixture was reduced in vacuum and purified by MPLC (eluent: MeOH/H2O 7:3).
General procedure for the deprotection of the acyclic amino acid derivatives II (GP9b)
12 M NaOH (2.0 eq) was added to a solution of the protected compound (1.0 eq) in MeOH (15.0 ml). After stirring for 16 h at rt, the mixture was freeze-dried. 1,2-Diaminoethane (7.0 eq) was added and the mixture was heated in a microwave for 16 h at 140 °C. Finally, the reaction mixture was reduced in vacuum and purified by MPLC (eluent: MeOH/H2O 7:3).
3-(Butylamino)-2-hydroxypropanoic acid (7b)
GP4 was followed using 7a (90 mg, 0.86 mmol), EtOH (1.0 ml), KOH (114 mg, 1.72 mmol), 1-bromobutane (106 mg, 0.774 mmol). The desired compound was obtained as amorphous white solid (110 mg, 79%). 1H NMR (400 MHz, MeOD) δ = 4.07 (dd, J = 8.1, 4.7 Hz, 1H, COOHCH), 3.24 (dd, J = 12.5, 4.7 Hz, 1H, COOHCHCH2), 3.06 (dd, J = 12.5, 8.1 Hz, COOHCHCH2), 3.04–2.99 (m, 2H, CH3CH2CH2CH2), 1.74–1.57 (m, 2H, CH3CH2CH2), 1.43 (m, 2H, CH3CH2CH2), 0.99 (t, J = 7.4 Hz, 3H, CH3CH2CH2) ppm; 13C NMR (101 MHz, MeOD) δ = 177.2 (COOH), 68.9 (COOHCH), 52.2 (COOHCHCH2), 48.6 (NCH2CH2CH2CH3), 29.2 (NCH2CH2CH2CH3), 20.8 (NCH2CH2CH2CH3), 13.9 (NCH2CH2CH2CH3) ppm; IR (KBr): ṽ = 3397, 1463, 1390, 1135, 1110, 770 cm−1; HRESIMS m/z (pos): 162.1123 C7H16NO3 (calcd. 162.1130).
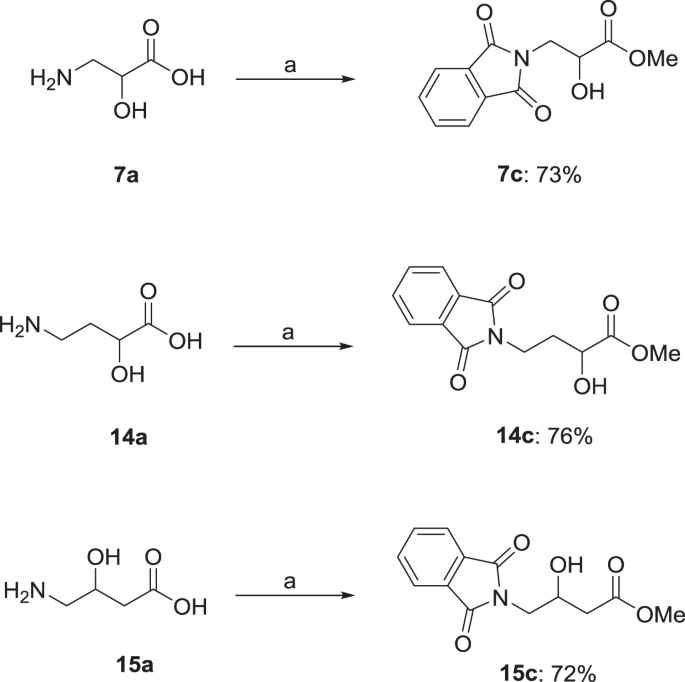
Methyl 3-(1,3-dioxoisoindolin-2-yl)-2-hydroxypropanoate (7c)
GP5b was followed using 3-amino-2-hydroxypropionic acid 7a (1.10 g, 10.5 mmol), phthalic anhydride (1.56 g, 10.5 mmol) in MeOH (80.0 ml) and 2 M HCl in Et2O (80.0 ml). The desired compound was obtained as amorphous white solid (2.00 g, 76%). 1H NMR (400 MHz, CDCl3) δ = 7.90–7.83 (m, 2H, NCOCarCHarCHar), 7.78–7.69 (m, 2H, NCOCarCHarCHar), 4.50 (td, J = 6.5, 5.1 Hz, 1H, NCH2CH), 4.08 (dd, J = 14.1, 5.1 Hz, 1H, NCH2), 4.02 (dd, J = 14.1, 6.4 Hz, 1H, NCH2), 3.82 (s, 3H, OCH3), 3.07 (d, J = 6.8 Hz, 1H, OH) ppm; 13C NMR (101 MHz, CDCl3) δ = 173.2 (COOCH3), 168.4 (NCO), 134.3 (NCOCarCHarCHar), 132.0 (NCOCarCHarCHar), 123.7 (NCOCarCHarCHar), 68.7 (NCH2CH), 53.2 (OCH3), 41.2 (NCH2) ppm; IR (HBr): ṽ = 3497, 1747, 1700, 1464, 1440, 1396, 1310, 1231, 1095, 983, 883, 722 cm−1; HRESIMS: m/z (pos): 272.0529 C12H11NO5Na (calcd. 272.0535).
Methyl 3-{2-[(tert-butyldiphenylsilyl)oxy]ethoxy}-3-{-(1,3-dioxoisoindolin-2-yl})propanoate (7d)
GP6 was followed using 7c (4.3 mmol, 1072 mg), tert-butyl(2-iodoethoxy)diphenylsilane (6.02 mmol, 2374 mg), Ag2CO3 (17.2 mmol, 4743 mg), toluene (15.0 ml), 4d. The desired compound was obtained as yellow oil (1882 mg, 82%). 1H NMR (500 MHz, CD2Cl2) δ = 7.85–7.78 (m, 2H, NCOCarCHarCHar), 7.76–7.68 (m, 2H, NCOCarCHarCHar), 7.66–7.57 (m, 4HrCHar), 7.47–7.33 (m, 6H, CHar), 4.38 (dd, J = 6.8, 6.0 Hz, 1H, NCH2CH), 4.02 (dd, J = 14.0, 6.8 Hz,1H, NCH2), 3.98 (dd, J = 14.0, 6.0 Hz, 1H, NCH2), 3.78–3.68 (m, 6H, CHOCH2CH2Si, OCH3), 3.58–3.49 (m, 1H, CHOCH2CH2Si), 0.94 (s, 9H, CCH3) ppm; 13C NMR (126 MHz, CD2Cl2) δ = 171.3 (COOCH3), 168.4 (NCO), 136.1 (Car), 134.6 (Car), 134.0 (Car), 132.5 (Car), 130.2 (Car), 128.2 (Car), 123.8 (Car), 76.7 (NCH2CH), 72.4 (OCH2CH2Si), 63.9 (OCH2CH2Si), 52.7 (OCH3), 39.9 (NCH2CH), 27.0 (SiCCH3), 19.4 (SiC) ppm; IR (film): ṽ = 2930, 1755, 1700, 1427, 1395, 1208, 1111, 703 cm−1; HRESIMS m/z (pos): 554.1970 C30H33NO6SiNa (calcd. 554.1975).
Methyl 3-(1,3-dioxoisoindolin-2-yl)-3-(2-hydroxyethoxy)propanoate (7e)
GP7 was followed using 7d (737 mg, 1.35 mmol), THF/pyridine 9:1 (10.0 ml), HF-pyridine (1.93 g, 6.75 mmol). The desired compound was obtained as a yellow oil (350 mg, 84%). 1H NMR (400 MHz, CD2Cl2) δ = 7.89–7.82 (m, 2H, NCOCarCHarCHar), 7.81–7.72 (m, 2H, NCOCarCHarCHar), 4.25 (dd, J = 6.5, 5.5 Hz, 1H, NCH2CH), 4.00 (dd, J = 14.0, 6.5 Hz, 1H, NCH2), 4.05 (dd, J = 14.0, 5.5 Hz, 1H, NCH2), 3.75 (s, 3H, OCH3), 3.70–3.54 (m, 4H, OCH2CH2OH) ppm; 13C NMR (101 MHz, CD2Cl2) δ = 171.55 (COOCH3), 168.6 (NCO), 134.8 (NCOCarCHarCHar), 132.5 (NCOCarCHar), 123.9 (NCOCarCHar), 76.9 (NCH2CH), 73.4 (OCH2CH2OH), 62.0 (OCH2CH2OH), 52.9 (OCH3), 40.0 (NCH2) ppm; IR (film): ṽ = 3474, 1774, 1770, 1429, 1396, 1213, 1029, 720 cm−1; HRESIMS m/z (pos): 294.0973 C14H16NO6 (calcd. 294.0978).
Methyl 3-{1,3-dioxoisoindolin-2-yl}-2-{2-[tris(4-methoxyphenyl)methoxy]ethoxy}propanoat (7f)
GP8 was followed using 7e (367 mg, 1.25 mmol), DMF (1 drop), pyridine (4.0 ml), 4,4′,4″-trimethoxytrithyl chloride (856 mg, 2.25 mmol). The desired compound was obtained as yellow oil (705 mg, 90%). 1H NMR (500 MHz, CD2Cl2) δ = 7.87–7.76 (m, 2H, NCOCarCHarCHar), 7.76–7.67 (m, 2H, NCOCarCHarCHar), 7.26–7.17 (m, 6H, CarCHarCHarCarOCH3), 6.81–6.68 (m, 6H, CarCHarCHarCarOCH3), 4.38 (dd, J = 7.3, 5.5 Hz, 1H, NCH2CH), 4.06 (dd, J = 14.0, 7.4 Hz, 1H, NCH2), 4.00 (dd, J = 14.1, 5.5 Hz, 1H, NCH2), 3.77 (s, 9H, CarOCH3), 3.74 (s, 3H, COOCH3), 3.73 (ddd, J = 10.5, 5.4, 3.5 Hz, 1H, OCH2CH2OCCar),3.52 (ddd, J = 10.4, 6.9, 3.5 Hz, 1H, OCH2CH2OCCar), 3.16 (ddd, J = 10.4, 6.9, 3.5 Hz, 1H, OCH2CH2OCCar), 3.06 (ddd, J = 10.3, 5.3, 3.5 Hz, 1H, OCH2CH2OCCar) ppm; 13C NMR (126 MHz, CD2Cl2) δ = 171.3 (COOCH3), 168.4 (NCO), 158.9 (CCarCHarCHarCarOCH3), 137.2 (CCarCHar), 134.6 (NCOCarCHarCHar), 132.5 (NCOCar), 130.2 (CH3OCarCHarCHar), 123.8 (NCOCarCHarCHar), 113.5 (CCarCHarCHarCarOCH3), 86.2 (OCH2CH2OCCar), 76.8 (NCH2CH), 70.9 (OCH2CH2OCCar), 63.5 (OCH2CH2OCCar), 55.7 (CarOCH3), 52.7 (COOCH3), 40.0 (NCH2CH) ppm; IR (film): ṽ = 1776, 1607, 1506, 1464, 1395, 1249, 1176, 1034, 827 cm−1; HRESIMS m/z (pos): 648.2210 C36H35NO9Na (calcd. 648.2210).
3-Amino-2-{2-[tris(4-methoxyphenyl)methoxy]ethoxy}propanoic acid (7g)
GP9a was followed using 7f (192 mg, 0.300 mmol), MeOH (3.0 ml), 12 M NaOH (0.05 ml), 1,2-diaminoethane (126 mg, 2.10 mmol). The desired compound was obtained as amorphous white solid (123 mg, 83%). 1H NMR (500 MHz, 0.1 M NaOD/MeOD) δ = 7.46–7.14 (m, 6H, CH3OCarCHarCHar), 7.05–6.65 (m, 6H, CH3OCarCHarCHar), 3.85 (ddd, J = 10.6, 5.2, 4.4 Hz, 1H, OCH2CH2OCCar), 3.81 (dd, J = 6.4, 3.8 Hz, 1H, NCH2CH), 3.77 (s, 9H, OCH3), 3.52 (ddd, J = 10.9, 6.7, 4.1 Hz, 1H, OCH2CH2OCCar), 3.31 (m, 1H, OCH2CH2OCCar), 3.24 (ddd, J = 10.0, 5.4, 4.1 Hz, 1H, OCH2CH2OCCar), 2.93 (dd, J = 13.4, 3.8 Hz, 1H, NCH2), 2.85 (dd, J = 13.4, 6.4 Hz, 1H, NCH2) ppm; 13C NMR (126 MHz, 1 M NaOD/MeOD) δ = 180.8 (COOH), 179.2 (CH3OCarCHarCHarCar), 159.9 (CH3OCarCHarCHarCar), 138.0 (CH3OCarCHarCHarCar), 131.0 (CH3OCarCHarCHarCar), 87.3 (OCH2CH2OCCar), 84.3 (NCH2CH), 70.5 (OCH2CH2OCCar), 64.4 (OCH2CH2OCCar), 55.9 (OCH3), 45.2 (NCH2CH) ppm; IR (HBR): ṽ = 3375, 1608, 1508, 1420, 1303, 1176, 1034 cm−1; HRESIMS m/z (neg): 480.2030 C27H30NO7 (calcd. 480.2028).
1-Butyl-3-hydroxyazetidine-3-carboxamide (11c)
GP2 was followed using 1-benzhydryl-3-hydroxyazetidine-3-carboxamide 11b (350 mg, 1.20 mmol), palladium on charcoal (10% Pd, 131 mg, 0.120 mmol) and butyraldehyde (0.28 ml, 3.1 mmol) in EtOH (2.0 ml). The crude product was purified by flash column chromatography on silica (eluent: EtOAc/MeOH 95:5 + 3% triethylamine) to afford the desired compound as amorphous white solid (177 mg, 83%). 1H NMR (400 MHz, MeOD): δ (ppm) = 3.65–3.55 (m, 2H, NCH2COH), 3.27–3.22 (m, 2H, NCH2COH), 2.57–2.48 (m, 2H, CH3CH2CH2CH2N), 1.41–1.28 (m, 4H, CH3CH2CH2CH2N + CH3CH2CH2CH2N), 0.96–0.87 (m, 3H, CH3CH2CH2CH2N); 13C NMR (101 MHz, MeOD): δ (ppm) = 14.4 (CH3CH2CH2CH2N), 21.5 (CH3CH2CH2CH2N), 30.6 (CH3CH2CH2CH2N), 59.8 (CH3CH2CH2CH2N), 65.6 (NCH2COH), 72.2 (NCH2COH), 178.4 (CONH2); IR (KBr): ν̃ = 3467, 2930, 2361, 1694, 1383, 1252, 1164, 899, 765, 669 cm−1; HRESIMS m/z (pos): 173.1288 C8H17N2O2 (calcd. 173.1285).
1-Butyl-3-hydroxyazetidine-3-carboxylic acid (11d)
GP3 was followed using 1-butyl-3-hydroxyazetidine-3-carboxamide 11c (88 mg, 0.51 mmol) and barium hydroxide octahydrate (380 mg, 1.20 mmol) in EtOH/H2O 1:1 (10.0 ml), 24 h, reflux. The desired compound was obtained as amorphous white solid (84 mg, 95%). 1H NMR (400 MHz, D2O + NaOD): δ (ppm) = 3.58 (d, J = 9.2 Hz, 2H, NCH2COH), 3.21 (d, J = 9.1 Hz, 2H, NCH2COH), 2.54–2.48 (m, 2H, CH3CH2CH2CH2N), 1.36–1.20 (m, 4H, CH3CH2CH2CH2N + CH3CH2CH2CH2N), 0.89–0.82 (m, 3H, CH3CH2CH2CH2N); 13C NMR (101 MHz, D2O + NaOD): δ (ppm) = 180.5 (COO), 72.6 (NCH2COH), 64.7 (NCH2COH), 58.8 (CH3CH2CH2CH2N), 29.4 (CH3CH2CH2CH2N), 20.5 (CH3CH2CH2CH2N), 13.4 (CH3CH2CH2CH2N); IR (KBr): ν̃ = 3467, 2930, 2361, 1694, 1383, 1252, 1164, 899, 765, 669 cm−1; HRESIMS m/z (pos): 173.1129 C8H16NO3 (calcd. 174.1125).
Ethyl 2-(1-benzhydryl-3-hydroxyazetidin-3-yl)acetate (11e)
GP1 was followed using LiHMDS in methyl tert-butyl ether (2.0 ml, 1.9 mmol), EtOAc (0.19 ml, 1.9 mmol) and 1-benzhydrylazetidin-3-one 11a (424 mg, 1.70 mmol) in THF (2.0 ml). The crude product was purified by flash column chromatography on silica (eluent: pentane/Et2O 3:1 + 2% diethylmethylamine) to afford the desired compound as amorphous white solid (448 mg, 80%).1H NMR (500 MHz, CDCl3): δ (ppm) = 7.47–7.11 (m, 10H, CHar), 4.39 (s, 1H, NCHCar), 4.17 (q, J = 7.1 Hz, 2H, COOCH2CH3), 3.79 (s, 1H, OH), 3.34–3.17 (m, 2H, NCH2COH), 3.09–2.98 (m, 2H, NCH2COH), 2.91 (s, 2H, CH2COO), 1.28 (t, J = 7.2 Hz, 3H, COOCH2CH3); 13C NMR (101 MHz, CDCl3): δ (ppm) = 172.9 (COOCH2CH3), 142.3 (NCHCCHCHCH), 128.5 (NCHCCHCHCH), 127.6 (NCHCCHCHCH), 127.3 (NCHCCHCHCH), 77.9 (NCHCar), 67.6 (NCH2COH), 65.2 (NCH2COH), 61.1 (COOCH2CH3), 43.0 (CH2COO), 14.3 (COOCH2CH3); IR (KBr): ν̃ = 3404, 2952, 1718, 1451, 1304, 1231, 1080, 902, 749, 706 cm−1; HREIMS m/z (pos): 325.1662 C20H23NO3 (calcd. 325.1672).
Ethyl 2-(1-butyl-3-hydroxyazetidin-3-yl)acetate (11f)
GP2 was followed using 11e (263 mg, 0.810 mmol), palladium on charcoal (10% Pd, 84 mg, 0.081 mmol) and butyraldehyde (0.18 ml, 2.0 mmol) in EtOH (5.0 ml). The crude product was purified by flash column chromatography on silica gel (eluent: Et2O + 3% triethylamine) to afford the desired compound as colourless oil (195 mg, 87%). 1H NMR (500 MHz, CDCl3): δ (ppm) = 4.19 (q, 2H, J = 7.2 Hz, COOCH2CH3), 3.92 (br s, 1H, OH), 3.43–3.25 (m, 2H, NCH2COH), 3.14–2.96 (m, 2H, NCH2COH), 2.86 (s, 2H, CH2COO), 2.52–2.40 (m, 2H, NCH2CH2CH2CH3), 1.37–1.23 (m, 7H, NCH2CH2CH2CH3 + NCH2CH2CH2CH3 + COOCH2CH3), 0.98–0.71 (m, 3H, NCH2CH2CH2CH3); 13C NMR (126 MHz, CDCl3): δ (ppm) = 173.0 (COOCH2CH3), 68.3 (COH), 66.3 (NCH2COH), 61.0 (COOCH2CH3), 59.7 (NCH2CH2CH2CH3), 42.8 (CH2COO), 30.1 (NCH2CH2CH2CH3), 20.6 (NCH2CH2CH2CH3), 14.3 (COOCH2CH3), 14.2 (NCH2CH2CH2CH3); IR (film): ν̃ = 3456, 2958, 2933, 1737, 1465, 1370, 1190, 1031, 948, 879 cm−1; HRESIMS m/z (pos): 216.1589 C11H22NO3 (calcd. 216.1594).
2-(1-Butyl-3-hydroxyazetidin-3-yl)acetic acid (11g)
GP3 was followed using 11f (34 mg, 0.16 mmol) and barium hydroxide octahydrate (201 mg, 0.630 mmol) in EtOH/H2O 1:1 (6.0 ml), 19 h, rt. The desired compound was obtained as amorphous white solid (26 mg, 87%). 1H NMR (400 MHz, D2O + NaOD): δ (ppm) = 3.39–3.33 (m, 2H, NCH2COH), 3.09–3.02 (m, 2H, NCH2COH), 2.61 (s, 2H, CCH2COO), 2.54–2.45 (m, 2H, CH3CH2CH2CH2N), 1.40–1.27 (m, 4H, CH3CH2CH2CH2N + CH3CH2CH2CH2N), 0.96–0.87 (m, 3H, CH3CH2CH2CH2N); 13C NMR (101 MHz, D2O + NaOD): δ (ppm) = 180.4 (COO), 69.1 (NCH2COH), 66.1 (NCH2COH), 59.6 (CH3CH2CH2CH2N), 46.1 (CH2COO), 29.8 (CH3CH2CH2CH2N), 20.9 (CH3CH2CH2CH2N), 14.3 (CH3CH2CH2CH2N); IR (film): ν̃ = 3427, 2930, 2360, 2342, 1583, 1569, 1420, 1108, 912, 686 cm−1; HREIMS m/z (pos): 187.1193 C9H17NO3 (calcd. 187.1203).
Ethyl 2-(1-benzyl-3-hydroxypyrrolidin-3-yl)acetate (12e)
GP1 was followed using LiHMDS in MTBE (2.1 ml, 2.0 mmol), EtOAc (0.20 ml, 2.0 mmol) and 1-benzylpyrrolidin-3-one 12a (371 mg, 2.10 mmol) in THF (1.0 ml). The crude product was purified by flash column chromatography on silica (eluent: pentane/EtOAc 6:4 + 2% diethylmethylamine) to afford the desired compound as pale-yellow oil (499 mg, 93%). 1H NMR (500 MHz, CDCl3): δ (ppm) = 7.26 (m, 5H, CHar), 4.17 (q, J = 7.1 Hz, 2H, COOCH2CH3), 3.64 (s, 2H, CarCH2N), 3.58 (br s, 1H, OH), 2.80–2.74 (m, 1H, NCH2CH2), 2.71–2.54 (m, 5H, NCH2CH2 + NCH2COH + NCH2COH + CH2COO), 2.01–1.94 (m, 1H, NCH2CH2), 1.93–1.85 (m, 1H, NCH2CH2), 1.26 (t, J = 7.1 Hz, 3H, COOCH2CH3); 13C NMR (126 MHz, CDCl3): δ (ppm) = 172.8 (COOCH2CH3), 139.0 (NCH2CCHCHCH), 128.9 (NCH2CCHCHCH), 128.4 (NCH2CCHCHCH), 127.1 (NCH2CCHCHCH), 66.6 (NCH2COH), 60.9 (COOCH2CH3), 60.3 (NCH2Car), 52.9 (NCH2CH2), 44.5 (CH2COO), 39.4 (NCH2CH2), 14.3 (COOCH2CH3); IR (film): ν̃ = 3512, 2978, 2796, 1732, 1372, 1189, 1029, 911, 740, 699 cm−1; HREIMS m/z (pos): 263.1525 C15H21NO3 (calcd. 263.1516).
Ethyl 2-(1-butyl-3-hydroxypyrrolidin-3-yl)acetate (12f)
GP2 was followed using 12e (290 mg, 1.10 mmol), palladium on charcoal (10% Pd, 117 mg, 0.110 mmol) and butyraldehyde (0.30 ml, 3.3 mmol) in EtOH (3.5 ml). The crude product was purified by flash column chromatography on silica gel (eluent: pentane/Et2O 7:3 + 3% diethylmethylamine) to afford the desired compound as colourless oil (195 mg, 77%).1H NMR (400 MHz, CDCl3): δ (ppm) = 4.18 (q, J = 7.2 Hz, 2H, COOCH2CH3), 3.54 (br s, 1H, OH), 2.81–2.61 (m, 4H, CH2COO + NCH2CH2COH + NCH2COH), 2.57–2.38 (m, 4H, NCH2CH2COH + NCH2COH + NCH2CH2CH2CH3), 2.01–1.92 (m, 1H, NCH2CH2COH), 1.86 (m, 1H, NCH2CH2COH), 1.51–1.40 (m, 2H, NCH2CH2CH2CH3), 1.38–1.25 (m, 5H, COOCH2CH3 + NCH2CH2CH2CH3), 0.91 (t, J = 7.3 Hz, 3H, NCH2CH2CH2CH3); 13C NMR (126 MHz, CDCl3): δ (ppm) = 172.8 (COOCH2CH3), 77.6 (COH), 67.0 (NCH2COH), 60.9 (COOCH2CH3), 56.2 (NCH2CH2CH2CH3), 53.1 (NCH2CH2COH), 44.6 (CH2COO), 39.4 (NCH2CH2COH), 30.9 (NCH2CH2CH2CH3), 20.9 (NCH2CH2CH2CH3), 14.3 (COOCH2CH3), 14.2 (NCH2CH2CH2CH3); IR (film): ν̃ = 3442, 2958, 2797, 1735, 1465, 1370, 1190, 1031, 946, 904 cm−1; HREIMS m/z (pos): 229.1673 C12H23NO3 (calcd. 229.1672).
2-(1-Butyl-3-hydroxypyrrolidin-3-yl)acetic acid (12g)
GP3 was followed using 12f (181 mg, 0.790 mmol) and barium hydroxide octahydrate (500 mg, 1.58 mmol) in EtOH/H2O 1:1 (8.0 ml), 6 h, rt. The desired compound was obtained as amorphous off-white solid (153 mg, 96%). 1H NMR (400 MHz, D2O + NaOD): δ (ppm) = 3.90–3.66 (m, 2H, NCH2COH + NCH2CH2COH), 3.46–3.14 (m, 4H, NCH2COH + NCH2CH2COH + NCH2CH2CH2CH3), 2.80–2.59 (m, 2H, CH2COO), 2.36–2.03 (m, 2H, NCH2CH2COH + NCH2CH2COH), 1.78–1.62 (m, 2H, NCH2CH2CH2CH3), 1.39 (h, J = 7.4 Hz, 2H, NCH2CH2CH2CH3), 0.93 (t, J = 7.4 Hz, 3H, NCH2CH2CH2CH3); 13C NMR (101 MHz, D2O + NaOD): δ (ppm) = 180.2 (COOCH2CH3), 78.1 (COH), 65.7 (NCH2COH), 55.9 (NCH2CH2CH2CH3), 52.7 (NCH2CH2COH), 47.1 (CH2COO), 38.5 (NCH2CH2COH), 29.7 (NCH2CH2CH2CH3), 20.3 (NCH2CH2CH2CH3), 13.4 (NCH2CH2CH2CH3); IR (KBr): ν̃ = 3426, 2965, 2876, 2360, 1589, 1395, 1094, 1014, 741, 669 cm−1; HRESIMS m/z (pos): 202.1439 C10H20NO3 (calcd. 202.1438).
1-Benzyl-3-hydroxypiperidine-3-carboxamide (13b)
1-Benzylpiperidin-3-one hydrochloride 13a (571 mg, 2.50 mmol) was solved in dry CH2Cl2 (4.0 ml). Freshly distilled triethylamine (0.85 ml, 6.1 mmol) was added and the brownish suspension was placed in an ultrasound bath for 15 min. After addition of trimethylsilyl cyanide (0.80 ml, 6.3 mmol) the reaction mixture was stirred for 48 h, diluted with CH2Cl2 (10.0 ml), filtered through a paper filter and reduced in vacuum. The oily residue was solved in CH2Cl2 (7.0 ml) and cooled to 0 °C. Concentrated sulfuric acid (0.70 ml, 13 mmol) was added and the biphasic mixture was stirred for 2 h at rt, after which the mixture was cooled to 0 °C, diluted with H2O (5.0 ml) and alkalized with 25% ammonium hydroxide solution. Potassium sodium tartrate (0.50 g) was added and the mixture was extracted five times with CH2Cl2 (20.0 ml). The combined organic phases were dried over MgSO4 and reduced in vacuum. The crude product was purified by flash column chromatography on silica (eluent: ethyl acetate + 3% triethylamine) to afford the desired compound as amorphous off-white solid (567 mg, 96%).1H NMR (400 MHz, 1,1,2,2-tetrachloroethane-d2, 80 °C): δ (ppm) = 7.61 (br s, 1H, NH2), 7.44–7.29 (m, 5H, CHar), 5.46 (br s, 1H, NH2), 3.92 (br s, 1H, OH), 3.64 (s, 2H, NCH2Car), 2.74 (dd, J = 11.3, 1.0 Hz, 1H, NCHaxHeqCOH), 2.63–2.46 (m, 3H, NCHaxHeqCOH + NCHaxHeqCH2CH2 + NCHaxHeqCH2CH2), 1.96 (dddd, J = 13.0, 7.8, 5.1, 1.0 Hz, 1H, NCH2CH2CHaxHeq), 1.89–1.57 (m, 3H, NCH2CH2CHaxHeq + NCH2CHaxHeqCH2 + NCH2CHaxHeqCH2); 13C NMR (101 MHz, 1,1,2,2-tetrachloroethane-d2, 80 °C): δ (ppm) = 176.8 (CNH2), 137.2 (NCH2CCHCHCH), 128.9 (NCH2CCHCHCH), 128.3 (NCH2CCHCHCH), 127.3 (NCH2CCHCHCH), 71.9 (NCH2COH), 62.6 (NCH2Car), 60.3 (NCH2COH), 52.7 (NCH2CH2CH2), 33.6 (NCH2CH2CH2), 21.5 (NCH2CH2CH2); IR (KBr): ν̃ = 3457, 3409, 2790, 1663, 1274, 1206, 1020, 924, 735, 665 cm−1; HREIMS m/z (pos): 234.1361 C13H18N2O2 (calcd. 234.1363).
1-Butyl-3-hydroxypiperidine-3-carboxamide (13c)
GP2 was followed using 1-benzyl-3-hydroxypiperidine-3-carboxamide 13b (300 mg, 1.30 mmol), palladium on charcoal (10% Pd, 138 mg, 0.130 mmol) and butyraldehyde (0.29 ml, 3.20 mmol) in EtOH (3.5 ml). The crude product was purified by flash column chromatography on silica (eluent: EtOAc + 3% triethylamine) to afford the desired compound as amorphous white solid (238 mg, 93%). 1H NMR (400 MHz, 1,1,2,2-tetrachloroethane-d2, 80 °C): δ (ppm) = 7.65 (br s, 1H, NH2), 5.35 (br s, 1H, NH2), 3.96 (br s, 1H, OH), 2.63 (d, J = 11.4 Hz, 1H, NCHaxHeqCOH), 2.48–2.28 (m, 5H, CH3CH2CH2CH2N + NCHaxHeqCOH + NCHaxHeqCH2CH2OH + NCHaxHeqCH2CH2OH), 1.86–1.78 (m, 1H, NCH2CH2CHaxHeqCOH), 1.76–1.57 (m, 2H, NCH2CHaxHeqCH2COH + NCH2CHaxHeqCH2COH), 1.54–1.37 (m, 3H, CH3CH2CH2CH2N + NCH2CH2CHaxHeqCOH), 1.34–1.20 (m, 2H, CH3CH2CH2CH2N), 0.88 (t, J = 7.3 Hz, 3H, CH3CH2CH2CH2N); 13C NMR (101 MHz, 1,1,2,2-tetrachloroethane-d2, 80 °C): δ (ppm) = 177.4 (CNH2), 72.1 (NCH2COH), 61.0 (NCH2COH), 58.1 (CH3CH2CH2CH2N), 53.1 (NCH2CH2CH2COH), 34.2 (NCH2CH2CH2COH), 29.0 (NCH2CH2CH2OH), 21.4 (CH3CH2CH2CH2N), 14.1 (CH3CH2CH2CH2N);. IR (KBr): ν̃ = 3462, 2955, 2805, 1682, 1454, 1399, 1137, 1019, 917, 659 cm−1; HREIMS m/z (pos): 200.1519 C10H20N2O2 (calcd. 200.1525).
1-Butyl-3-hydroxypiperidine-3-carboxylic acid (13d)
GP3 was followed using 1-butyl-3-hydroxypiperidine-3-carboxamide 13c (80 mg, 0.40 mmol) and barium hydroxide octahydrate (254 mg, 0.801 mmol) in EtOH/H2O 1:1 (10.0 ml), 5 h, reflux. The desired compound was obtained as colourless semi-solid (76 mg, 94%). 1H NMR (400 MHz, D2O + NaOD): δ (ppm) = 2.49 (br d, J = 10.8 Hz, 1H, NCHaxHeqCH2CH2COH), 2.38 (d, J = 12.0 Hz, 1H, NCHaxHeqCOH), 2.06–1.90 (m, 3H, CH3CH2CH2CH2N + NCHaxHeqCOH), 1.72–1.61 (m, 1H, NCHaxHeqCH2CH2COH), 1.47–1.20 (m, 4 H, NCH2CH2CHaxHeqCOH + NCH2CH2CHaxHeqCOH + NCH2CHaxHeqCH2COH + NCH2CHaxHeqCH2COH), 1.15–1.03 (m, 2H, CH3CH2CH2CH2N), 0.92 (h, J = 7.3 Hz, 2H, CH3CH2CH2CH2N), 0.54 (t, J = 7.3 Hz, 3H, CH3CH2CH2CH2N); 13C NMR (101 MHz, D2O + NaOD): δ (ppm) = 182.1 (COO), 74.3 (NCH2COH), 59.6 (NCH2COH), 57.9 (CH3CH2CH2CH2N), 52.8 (NCH2CH2CH2COH), 32.2 (NCH2CH2CH2COH), 27.2 (CH3CH2CH2CH2N), 20.3 (NCH2CH2CH2COH), 20.2 (CH3CH2CH2CH2N), 13.3 (CH3CH2CH2CH2N); IR (KBr): ν̃ = 3414, 2961, 2874, 1609, 1388, 1179, 1120, 1016, 949, 719 cm−1; HREIMS m/z (pos): 201.1365 C10H19NO3 (calcd. 201.1359).
4-(Butylamino)-2-hydroxybutanoic acid (14b)
GP4 was followed using 14a (134 mg, 1,12 mmol), EtOH (2.4 ml), KOH (56,1 mg, 2.25 mmol), 1-bromobutane (139 mg, 1.01 mmol). The desired compound was obtained as amorphous white solid (155 mg, 79%). 1H NMR (500 MHz, MeOD) δ = 4.00 (t, J = 5.7 Hz, 1H,CHOH), 3.16–3.09 (m, 2H, OHCCH2CH2N), 2.97 (m, 2H, NCH2CH2CH2CH3), 2.05 (dtd, J = 14.2, 7.1, 5.5 Hz, 1H, OHCHCH2), 1.95 (dq, J = 14.5, 6.2 Hz, 1H, OHCHCH2), 1.65 (m, 2H, CH3CH2CH2), 1.43 (m, 2H, CH3CH2CH2), 0.99 (t, J = 7.4 Hz, 3H, CH3CH2CH2 ppm); 13C NMR (126 MHz, MeOD) δ = 180.0 (COOH), 72.0 (COOHCH), 49.8 (NCH2CH2CH2CH3), 46.8 (OHCHCH2CH2), 31.7 (OHCHCH2CH2), 29.4 (NCH2CH2CH2CH3), 20.8 (NCH2CH2CH2CH3), 13.9 (NCH2CH2CH2CH3) ppm; IR (KBr): ṽ = 3411, 1651, 1359, 1335, 1103, 820 cm−1; HRESIMS m/z (pos): 176.1281 C8H18NO3 (calcd. 176.1287).
Methyl 2-{2-[(tert-butyldiphenylsilyl)oxy]ethoxy}-4-(1,3-dioxoisoindolin-2-yl)butanoate (14d)
GP6 was followed using 14c (369 mg, 1.20 mmol), tert-butyl(2-iodoethoxy)diphenylsilane (805 mg, 1.70 mmol), Ag2CO3 (1.32 g, 4.80 mmol), toluene (2.0 ml), 4d. The desired compound was obtained as yellow oil (500 mg, 76%). 1H NMR (500 MHz, CD2Cl2) δ = 7.83–7.77 (m, 2H, NCOCarCHarCHar), 7.73–7.66 (m, 6H, CHar), 7.47–7.36 (m, 6H, CHar), 4.02 (dd, J = 8.8, 3.8 Hz, 1H, NCH2CH2CH), 3.86–3.70 (m, 5H, NCH2, SiOCH2CH2), 3.68 (s, 3H, COOCH3), 3.54 (ddd, J = 9.7, 6.2, 4.2 Hz, 1H, SiOCH2CH2), 2.11 (dtd, J = 14.4, 7.3, 3.8 Hz, 1H, NCH2CH2), 2.08–2.00 (m, 1H, NCH2CH2), 1.04 (s, 9H, CCH3) ppm; 13C NMR (126 MHz, CD2Cl2) δ = 172.9 (COOCH3), 168.7 (NCO), 136.2 (Car), 134.5 (Car), 134.2 (Car), 132.7 (Car), 130.2 (Car), 128.2 (Car), 123.6 (Car), 77.8 (NCH2CH2CH), 72.4 (SiOCH2CH2), 63.9 (SiOCH2CH2), 52.3 (COOCH3), 35.1 (NCH2), 32.2 (NCH2CH2), 27.1 (CCH3), 19.6 (CCH3) ppm; IR (film): ṽ = 2932, 1770, 1468, 1396, 1112, 823, 738, 703 cm−1; HRESIMS m/z (pos): 568.2125 C31H35NO6SiNa (calcd. 568.2126).
Methyl 4-(1,3-dioxoisoindolin-2-yl)-2-(2-hydroxyethoxy)butanoate (14e)
GP7 was followed using 14d (442 mg, 0.810 mmol), THF/pyridine 9:1 (10.0 ml), HF-pyridine (116 mg, 4.05 mmol). The desired compound was obtained as yellow oil (190 mg, 76%). 1H NMR (500 MHz, CDCl3) δ = 7.93–7.80 (m, 2H, CarCHarCHar), 7.80–7.66 (m, 2H, CarCHarCHar), 3.99 (ddd, J = 13.9, 8.8, 5.0 Hz, 1H, NCH2), 3.92 (dd, J = 9.9, 3.2 Hz, 1H, NCH2CH2CH), 3.81 (dt, J = 14.0, 5.5 Hz, 1H, NCH2), 3.76–3.67 (m, 6H, OCH3, CHOCH2CH2OH), 3.63–3.56 (m, 1H, CHOCH2CH2OH), 2.94 (t, J = 5.7 Hz, 1H, OH), 2.19 (dddd, J = 14.3, 8.7, 5.5, 3.2 Hz, 1H, NCH2CH2), 2.08 (ddt, J = 14.9, 9.78, 5.25, 5.25 Hz, 1H, NCH2CH2) ppm; 13C NMR (126 MHz, CDCl3) δ = 173.0 (COOCH3), 168.6 (NCO), 134.3 (CarCHarCHar), 132.2 (CarCHarCHar), 123.5 (CarCHarCHar), 76.7 (NCH2CH2CH), 72.7 (CHOCH2CH2OH), 62.0 (CHOCH2CH2OH), 52.3 (OCH3), 34.5 (NCH2CH2), 32.0 (NCH2CH2) ppm; IR (film): ṽ = 3464, 1771, 1760, 1700, 1438, 1398, 1148, 721 cm−1; HRESIMS m/z (pos): 330.0946 C15H17NO6Na (calcd. 330.0948).
Methyl 4-{1, 3-dioxoisoindolin-2-yl}-2-{2-[tris(4-methoxyphenyl)methoxy]ethoxy}butanoate (14f)
GP8 was followed using 14e (461 mg, 1.50 mmol), DMF (1 drop), pyridine (4.0 ml), 4,4′,4″-trimethoxytrithyl chloride (1.03 g, 2.70 mmol). The desired compound was obtained as a yellow oil (789 mg, 82%). 1H NMR (400 MHz, CDCl3) δ = 7.75–7.80 (m, 2H, NCOCarCHarCHar), 7.73–7.64 (m, 2H, NCOCarCHarCHar), 7.37–7.28 (m, 6H, CH3OCarCHarCHar), 6.86–6.77 (m, 6H, CH3OCarCHarCHar), 4.04 (dd, J = 7.6, 5.0 Hz, 1H, NCH2CH2CH), 3.88 (t, J = 6.9 Hz, 2H, NCH2), 3.79 (s, 10H, CarOCH3, CHOCH2CH2), 3.72 (s, 3H, COOCH3), 3.59 (ddd, J = 10.3, 6.2, 4.4 Hz, 1H, CHOCH2CH2), 3.22 (ddd, J = 10.3, 6.2, 4.3 Hz, 1H, CHOCH2CH2), 3.14 (ddd, J = 10.2, 5.8, 4.4 Hz, 1H, CHOCH2CH2), 2.30–1.88 (m, 2H, NCH2CH2) ppm; 13C NMR (101 MHz, CDCl3) δ = 172.7 (COOCH3), 168.3 (NCO), 158.4 (CH3OCarCHarCHarC), 136.9 (CH3OCarCHarCHarC), 134.0 (NCOCarCHarCHar), 132.3 (NCOCarCHarCHar), 129.9 (CH3OCarCHarCHarC), 123.3 (NCOCarCHarCHar), 113.2 (CH3OCarCHarCHarC), 85.8 (OCCar), 77.6 (NCH2CH2CH), 70.5 (CHOCH2CH2), 63.2 (CHOCH2CH2), 55.3 (CarOCH3), 52.1 (COOCH3), 34.9 (NCH2CH2), 31.9 (NCH2CH2) ppm; IR (film): ṽ = 1748, 1700, 1607, 1508, 1398, 1249, 1176, 1034 cm−1; HRESIMS m/z (pos): 678.2091 C37H37NO9 (calcd. 678.210).
4-Amino-2-{2-[tris(4-methoxyphenyl)methoxy]ethoxy}butanoic acid (14g)
GP9a was followed using 14f (192 mg, 0.300 mmol), MeOH (3.0 ml), 12 M NaOH (0.05 ml), 1,2-diaminoethane (126 mg, 2.10 mmol). The desired compound was obtained as amorphous white solid (102 mg, 69%). 1H NMR (400 MHz, 0.1 M NaOD/MeOD) δ = 7.48–7.04 (m, 6H, CH3OCarCHarCHar), 6.99–6.66 (m, 6H, CH3OCarCHarCHar), 3.87 (dd, J = 8.1, 4.4 Hz, 1H, NCH2CH2CH), 3.77 (s, 10H, CHOCH2CH2OC, OCH3), 3.45 (ddd, J = 10.9, 7.2, 3.9 Hz, 1H, CHOCH2CH2OC), 3.27 (m, 1H, CHOCH2CH2OC), 3.19 (m, 1H, CHOCH2CH2OC), 2.85–2.68 (m, 2H, NCH2CH2), 1.96–1.74 (m, 2H, NCH2CH2) ppm; 13C NMR (101 MHz, 0.1 M NaOD/ MeOD) δ = 180.9 (COOH), 159.9 (CCarCHarCHarCOCH3), 138.1 (CCarCHarCHarCOCH3), 131.0 (CCarCHarCHarCOCH3), 114.1 (CCarCHarCHarCOCH3), 87.2 (CCarCHarCHarCOCH3), 82.1 (NCH2CH2CH), 70.4 (CHOCH2CH2C), 64.4 (CHOCH2CH2C), 55.8 (OCH3), 40.1 (NCH2CH2), 37.5 (NCH2CH2) ppm; IR (KBr): ṽ = 1606, 1505, 1463, 1249, 1175, 1034, 828, 735 cm−1; HRESIMS m/z (neg): 494.2185 C28H32NO7 (calcd. 494.2184).
4-(Butylamino)-3-hydroxybutanoic acid (15b)
GP4 was followed using 15a (238 mg, 2.00 mmol), EtOH (2.4 ml), KOH (264 mg, 4.00 mmol), 1-bromobutane (249 mg, 1.80 mmol). The desired compound was obtained as amorphous white solid (263 mg, 75%). 1H NMR (500 MHz, MeOD) δ = 4.11 (dtd, J = 8.1, 6.2, 3.9 Hz, 1H, NCH2CHCH2), 3.09 (dd, J = 12.5, 3.8 Hz, 1H, NCH2CHCH2), 3.02–2.93 (m, 3H, NCH2CHCH2, NCH2CH2CH2CH3), 2.46 (dd, J = 15.6, 6.2 Hz, 1H, COOHCH2), 2.42 (dd, J = 15.6, 6.2 Hz, 1H, COOHCH2), 1.75–1.54 (m, 2H, NCH2CH2CH2CH3), 1.43 (m, 2H, NCH2CH2CH2CH3), 0.99 (t, J = 7.4 Hz, 3H, NCH2CH2CH2CH3) ppm; 13C NMR (126 MHz, MeOD) δ = 178.7 (COOH), 65.9 (CHOH), 53.9 (OHCHCH2N) 49.2 (NCH2CH2CH2CH3), 43.9 (COOHCH2), 29.4 (NCH2CH2CH2CH3), 20.9 (NCH2CH2CH2CH3), 13.9 (NCH2CH2CH2CH3) ppm; IR (KBr): ṽ = 3223, 1629, 1564, 1359, 1251, 1074 cm−1; HRESIMS m/z (pos): 176.1280 C8H18NO3 (calcd. 176.1287).
Methyl 4-(1,3-dioxoisoindolin-2-yl)-3-hydroxybutanoate (15c)
GP5a was followed using 4-amino-3-hydroxybutanoic acid 15a (1.25 g, 10.3 mmol), phthalic anhydride (1.56 g, 10.3 mmol), MeOH (80.0 ml) and 2 M HCl in Et2O (80.0 ml). The desired compound was obtained as amorphous white solid (2.00 g, 74%). 1H NMR (500 MHz, CDCl3) δ = 7.83–7.91 (m, 2H, NCOCarCHarCHar), 7.80–7.70 (m, 2H, NCOCarCHarCHar), 4.37 (ddq, J = 8.56, 7.11, 4.53, 4.49 Hz, 1H, NCH2CHCH2), 3.89 (dd, J = 14.1, 7.1 Hz, 1H, NCH2CHCH2), 3.79 (dd, J = 14.1, 4.5 Hz, 1H, NCH2CHCH2), 3.72 (s, 3H, OCH3), 3.20 (d, J = 5.1 Hz, 1H, OH), 2.61 (dd, J = 16.5, 4.0 Hz, 1H, NCH2CHCH2), 2.53 (dd, J = 16.5, 8.3 Hz, 1H, NCH2CHCH2) ppm; 13C NMR (126 MHz, CDCl3) δ = 172.4 (COOCH3), 168.7 (NCO), 134.3 (NCOCarCHarCHar), 132.1 (NCOCarCHarCHar), 123.6 (NCOCarCHarCHar), 66.8 (NCH2CHCH2), 52.1 (OCH3), 43.0 (NCH2CHCH2), 39.0 (NCH2CHCH2) ppm; IR (KBr): ṽ = 3450, 1773, 1700, 1610, 1395, 1206, 1024, 717 cm−1; HRESIMS m/z (pos): 286.0687 C13H13NO5Na (calcd. 286.0691).
Methyl 3-{2-[(tert-butyldiphenylsilyl)oxy]ethoxy}-4-{-(1,3-dioxoisoindolin-2-yl})butanoate (15d)
GP6 was followed using 15c (369 mg, 1.20 mmol), tert-butyl(2-iodoethoxy)diphenylsilane (662 mg, 1.68 mmol), Ag2CO3 (1.32 g, 4.80 mmol), toluene (15 ml), 4d. The desired compound was obtained as a yellow oil (537 mg, 82.0%). 1H NMR (500 MHz, CDCl3) δ = 7.83–7.77 (m, 2H, NCOCarCHarCHar), 7.73–7.68 (m, 2H, NCOCarCHarCHar), 7.67–7.62 (m, 4H, CHar), 7.43–7.32 (m, 6H, CHar), 4.14 (dq, J = 7.4, 5.5 Hz, 1H, NCH2CHCH2), 3.90–3.84 (m, 2H, NCH2CHCH2), 3.78 (dt, J = 9.7, 4.9 Hz, 1H, OCH2CH2Si), 3.75–3.71 (m, 2H, OCH2CH2Si), 3.66 (dt, J = 9.6, 4.5 Hz, 1H, OCH2CH2Si), 3.61 (s, 3H, OCH3), 2.60 (dd, J = 16.1, 7.5 Hz, 1H, NCH2CHCH2), 2.55 (dd, J = 16.1, 5.5 Hz, 1H, NCH2CHCH2), 1.00 (s, 9H ppm, SiCCH3) ppm; 13C NMR (126 MHz, CDCl3) δ = 171.5 (COOCH3), 168.4 (NCO), 135.7 (Car), 134.1 (Car), 133.8 (Car), 132.1 (Car), 129.7 (Car), 127.7 (Car), 123.5 (Car), 74.5 (NCH2CHCH2), 71.5 (OCH2CH2OSi), 63.5 (OCH2CH2Si), 51.8 (OCH3), 40.3 (NCH2CHCH2), 38.6 (NCH2CHCH2), 26.9 (SiCCH3), 19.3 (SiC) ppm; IR (film): ṽ = 2932, 1774, 1770, 1469, 1428, 1396, 1361, 1281, 1192, 1112, 823, 738 cm−1; HRESIMS m/z (pos): 568.2132 C31H35NO6SiNa (calcd. 568.2131).
Methyl 4-(1,3-dioxoisoindolin-2-yl)-3-(2-hydroxyethoxy)butanoate (15e)
GP7 was followed using 15d (442 mg, 0.81 mmol), THF/pyridine 9:1 (10.0 ml), HF-pyridine (116 mg, 4.05 mmol). The desired compound was obtained as yellow oil (206 mg, 83%). 1H NMR (500 MHz, CD2Cl2) δ = 7.92–7.80 (m, 2H, NCOCarCHarCHar), 7.80–7.71 (m, 2H, NCOCarCHarCHar), 4.10 (dq, J = 9.0, 4.5 Hz, 1H, NCH2CHCH2), 3.87 (dd, J = 14.3, 4.9 Hz, 1H, NCH2CHCH2), 3.80 (dd, J = 14.3, 4.5 Hz, 1H, NCH2CHCH2), 3.73 (ddd, J = 10.3, 5.9, 3.1 Hz, 1H, OCH2CH2OH), 3,66 (s, 3H, OCH3), 3.65 (ddd, J = 10.3, 5.9, 2.8 Hz, 1H, OCH2CH2OH), 3.60 (ddd, J = 12.3, 6.1, 2.8 Hz, 1H, OCH2CH2OH), 3.56 (ddd, J = 12.2, 6.0, 3.0, 1H, OCH2CH2OH), 2.76 (t, J = 6.3 Hz, 1H, OH), 2.59 (dd, J = 16.4, 4.3 Hz, 1H, NCH2CHCH2), 2.52 (dd, J = 16.4, 8.7 Hz, 1H, NCH2CHCH2) ppm; 13C NMR (126 MHz, CD2Cl2) δ = 172.3 (COOCH3), 169.1 (NCO), 134.7 (NCOCarCHarCHrar), 132.5 (NCOCarCHarCHar), 123.8 (NCOCarCHarCHar), 75.3 (NCH2CHCH2), 72.6 (OCH2CH2OH), 62.3 (OCH2CH2OH), 52.3 (OCH3), 40.9 (NCH2CHCH2), 38.6 (NCH2CHCH2) ppm; IR (film): ṽ = 3472, 2951, 1773, 1770, 1467, 1397, 112, 725 cm−1; HRESIMS m/z (pos): 330.0949 C15H17NO6Na (calcd. 330.0954).
Methyl 4-{1,3-dioxoisoindolin-2-yl}-3-{2-[tris(4-methoxyphenyl)methoxy]ethoxy}butanoate (15f)
GP8 was followed using 15e (338 mg, 1.10 mmol), DMF (1 drop), pyridine (4.0 ml), 4,4′,4″-trimethoxytrithyl chloride (753 mg, 1.98 mmol). The desired compound was obtained as yellow oil (520 mg, 74%). 1H NMR (400 MHz, CD2Cl2) δ = 7.89–7.76 (m, 2H, NCOCarCHarCHar), 7.76–7.65 (m, 2H, NCOCarCHarCHar), 7.32–7.18 (m, 6H, CH3OCarCHarCHar), 6.86–6.72 (m, 6H, CH3OCarCHarCHar), 4.13 (dq, J = 7.2, 5.6 Hz, 1H, NCH2CHH2), 3.89 (dd, J = 14.1, 5.5 Hz, 1H, NCH2CHCH2), 3.81 (dd, J = 14.0, 5.7 Hz, 1H, NCH2CHH2), 3.77 (s, 9H, CarOCH3), 3.76 (ddd, J = 10.2, 5.6, 4.4 Hz, 1H, OCH2CH2OCCar), 3.64 (ddd, J = 10.2, 5.6, 4.5 Hz, 1H, OCH2CH2OCCar), 3.59 (s, 3H, COOCH3), 3.10 (ddd, J = 12.2, 5.6, 4.3 Hz, 1H, OCH2CH2OCCar), 3.07 (ddd, J = 12.2, 5.6, 4.2 Hz, 1H, OCH2CH2OCCar), 2.62 (dd, J = 16.0, 7.1 Hz, 1H, NCH2CHCH2), 2.56 (dd, J = 16.0, 5.4 Hz, 1H, NCH2CHCH2) ppm; 13C NMR (101 MHz, CD2Cl2) δ = 171.8 (COOCH3), 168.8 (NCO), 158.9 (CH3OCar), 137.4 (CCarCHar), 134.6 (NCOCarCHarCHar), 132.6 (NCOCar), 130.3 (CH3OCarCHarCHar), 123.7 (NCOCarCHarCHar), 113.5 (CH3OCarCHarCHar), 86.1 (CCarCHarCHar), 75.1 (NCH2CHCH2), 70.3 (OCH2CH2OCCar), 63.7 (OCH2CH2OCCar), 55.7 (CarOCH3), 52.1 (COOCH3), 41.0 (NCH2CHCH2), 38.9 (NCH2CHCH2) ppm; IR (film): ṽ = 1773, 1607, 1507, 1396, 1249, 1175, 1033, 828 cm−1; HRESIMS m/z (pos): 662.2365 C37H37NO9Na (calcd. 662.2366).
4-Amino-3-{2-[tris(4-methoxyphenyl)methoxy]ethoxy}butanoic acid (15g)
GP9b was followed using 15f (141 mg, 0.220 mmol), MeOH (3.0 ml), 12 M NaOH (0.05 ml), 1,2-diaminoethane (92,6 mg, 1.54 mmol). The desired compound was obtained as amorphous white solid (75 mg, 69%). 1H NMR (400 MHz, 0.1 M NaOD/MeOD) δ = 7.36–7.21 (m, 6H, CH3OCarCHarCHar), 6.90–6.69 (m, 6H, CH3OCarCHarCHar), 3.82–3.72 (m, 11H, CH2CHCH2, OCH3, CHOCH2CH2OC), 3.63 (ddd, J = 10.35, 6.16, 3.94 Hz, 1H, CHOCH2CH2OC), 3.28–3.09 (m, 2H, CHOCH2CH2OC), 2.81 (dd, J = 13.4, 3.5 Hz, 1H, NCH2CHCH2), 2.64 (dd, J = 13.4, 7.2 Hz, 1H, NCH2CHCH2), 2.51 (dd, J = 14.2, 6.1 Hz, 1H, NCH2CHCH2), 2.25 (dd, J = 14.2, 7.3 Hz, 1H, NCH2CHCH2) ppm; 13C NMR (101 MHz, 0.1 M NaOD/MeOD) δ = 180.2 (COOH), 159.8 (CCarCHarCHarCOCH3), 138.0 (CCarCHarCHarCOCH3), 130.9 (CCarCHarCHarCOCH3), 114.1 (CCarCHarCHarCOCH3), 87.1 (CCarCHarCHarCOCH3), 80.8 (NCH2CHCH2), 70.1 (CHOCH2CH2C), 64.6 (CHOCH2CH2C), 56.0 (, OCH3), 46.2 (NCH2CH), 42.2 (NCH2CHCH2) ppm; IR (KBr): ṽ = 3375, 1606, 1505, 1463, 1249, 1175, 1034, 828, 735 cm−1; HRESIMS m/z (neg): 494.2196 C28H33NO7 (calcd. 494.2184).
Biological evaluation
HEK cells
HEK293 cells were purchased from the American Type Culture Collection (ATCC) specified as ATCC-CRL-1573 (Lot 57954093). The stably mGAT1, mGAT2, mGAT3 or mGAT4 expressing HEK293 cell lines were generated as described previously (Kragler et al. 2005, 2008). These cell lines were cultivated till reaching confluencies >85% (up to passage 25) and immediately used after detachment and washing.
[3H]GABA uptake assays
The [3H]GABA uptake assays were performed as reported (Kragler et al. 2005, 2008) in a 96‐well plate format with intact HEK293 cells expressing mGAT1, mGAT2, mGAT3 and mGAT4, respectively.
MS Binding Assays
The MS Binding Assays were performed as reported (Zepperitz et al. 2006) with mGAT1 membrane preparations obtained from a stable HEK293 cell line and NO711 as unlabelled marker in competitive binding experiments.
Results and discussion
Synthesis
Synthesis of the cyclic N-butylamino acid derivatives
For the synthesis of the cyclic N-butylhydroxyamino acids 11d, 11g, 12g, and 13d we intended to start from the benzyl- or benzhydryl-protected cyclic aminoketones 11a, 12a and 13a, respectively. Reaction of 11a and 13a with tetramethylsilyl cyanide followed by acidic hydrolysis of the thus to be formed TMS-protected cyanohydrines should furnish the corresponding α-hydroxycarboxamides 11b and 13b. Likewise, reaction of the cyclic aminoketones 11a and 12a with lithium 1-ethoxyethen-1-olate should lead to the β-hydroxyesters 11e and 12e. Deprotection of the amino nitrogen atom of 11b, 11e, 12e, and 13b, followed by introduction of a n-butyl rest via reductive amination and hydrolysis of the carboxamide and ester function, respectively, should finally furnish the desired free amino acids 11d, 11g, 12g, and 13d.
Synthesis of the cyclic α-hydroxycarboxamide 11b was performed according to literature (Lamb 2008). The same reaction sequence was applied for the synthesis of its ring-expanded analogue 13b. Hence, the N-benzylpyrrolid-3-one 13a was reacted with trimethylsilyl cyanide to give the respective TMS-protected cyanohydrine. Due to the lability of the TMS ether function, the TMS-protected cyanohydrine was not purified and characterized, but used for the next reaction step, the hydrolysis with concentrated sulfuric acid. This gave the corresponding α-hydroxycarboxmide 13b in an excellent yield of 96% over both reaction steps. The β-hydroxyesters 11e and 12e were obtained in good yields of 80–93% from the cyclic aminoketones 11a and 12a by reaction with lithium 1-ethoxyethen-1-olate, which was generated from ethyl acetate and LiHMDS at low temperature (Scheme 3).
Synthesis of the cyclic hydroxyamino acids 11d, 11g, 12d and 13d. Reagents and conditions: a 1. Trimethylsilyl cyanide (2.5 eq), CH2Cl2, rt, 48 h; 2. sulfuric acid (5.2 eq), CH2Cl2, 0 °C–rt, 2 h. b 1. LiHMDS (1.0 eq), ethyl acetate (1.0 eq), methyl tert-butyl ether, −78 °C, 25 min, 2. 11a/12a, THF, −78 °C to −10 °C. c H2 (10 bar), n-butyraldehyde (2.5 eq), 10% palladium on charcoal (0.1 eq), rt, 16 h; d barium hydroxide octahydrate (2.0–2.4 eq), EtOH/H2O 1:1, rt or reflux
Next, the carboxamide and ester derivatives 11b, 11e, 12e and 13b were subjected to deprotection of the nitrogen atom and subsequent n-butylation, accomplished in a single reaction step by exposing the compounds to hydrogen (10 bar) in presence of palladium on cabon and 2.0–2.4 equivalents of n-butyraldehyde. This furnished the respective N-butyl derivatives 11c, 11f, 12f, and 13c in yields of 77–93%. Finally, after basic hydrolysis and workup (barium hydroxide, carbon dioxide workup), the free amino acids 11d, 11g, 12g, and 13d were hence obtained in yields of 87–96% (Scheme 3).
Synthesis of the acyclic N-butylamino acid derivatives
For the synthesis of the N-butyl derivatives 7b, 14b, and 15b from the cyclic hydroxyamino acids 7a, 14a, and 15a, a specialized procedure developed for the monobutylation of β-alanine (Santimukul and Perez 2011) was followed. When according to this procedure 7a, 14a, and 15a were treated with n-bromobutane in a mixture of methanol and water under reflux the desired test compounds 7b, 14b, and 15b were obtained in yields of 75–79% (Scheme 4).
Synthesis of O-alkylated hydroxyamino acid derivatives
Since the unsubstituted hydroxyamino acids 7a, 14a, and 15a had been found to exhibit higher inhibitory potencies at all GAT subtypes than the corresponding N-butyl derivatives 7b, 14b, and 15b (see chapter 3 “biological evaluation”, Table 3), also the respective amino acid derivatives with an tris(4-methoxyphenyl)methyloxyethyl attached to the hydroxy function of the parent compounds should be included in this study.
As starting compounds for this synthesis of the target compounds 7g, 14g, and 15g, the derivatives 7c, 14c, and 15c seemed well suited, as they should allow a selective functionalization of the OH group as the carboxylic acid and the amino moieties in 7g, 14g, and 15g are protected in form of ester and phthalimidic moieties, respectively. The preparation of 14c has been described in literature (Farkas et al. 2009), the synthesis of 7c and 15c should be accomplished in an analogous manner. Based on these starting materials, 7c, 14c, and 15c, in the next steps first a 2-hydroxyethyl residue should be attached to the free OH function to serve as linker to the lipophilic domain of the target compound. The trityl based lipophilic moiety should be introduced only after that, as the reaction conditions required for the formation of the first ether function were thought to cause side reactions if the terminal trityl moiety were already present. Deprotection of the carboxylic acid and the amino group in 7f, 14f, and 15f should finally lead to the target compounds 7g, 14g, and 15g.
The synthesis of 14c was accomplished according to literature (Farkas et al. 2009) by first reacting 14a with phthalic anhydride to protect the terminal amino group as phthalimide moiety, followed by etherification of the carboxyl group to give the corresponding carboxylic acid ester, the overall product being 14c. Applying the same procedure to 7a and 15a, the analogous compounds 7c and 15c could be obtained in yields of 73% (7c) and 72% (15c) (Scheme 5).
The required hydroxylethylation of the OH function could be realized by treatment of 7c, 14c, and 15c with 2-iodoethoxy-TBDPS in the presence of Ag2CO3 furnishing the corresponding ethers 7d, 14d, and 15d in good yields of 76–82%. Removal of the TBDPS protecting group by treatment with HF-pyridine yielded in the free alcohols 7e, 14e, and 15e (76–84%), which upon reaction with 4,4′,4″-trimethoxytrityl chloride, gave the trityl derivatives 7f, 14f, and 15f (74–90%, Scheme 6).
Synthesis of acyclic amino acid derivatives comprising a 4,4′,4″-trimethoxytrityl moiety, which is linked to the 2- or 3-position of the carbon chain via an OCH2CH2O spacer (7g, 14g and 15g). Reagents and conditions: a Ag2CO3 (4.0 eq), tert-butyl(2-iodoethoxy)diphenylsilane (1.4 eq), toluene, reflux; b HF-pyridine (5.0 eq), THF/pyridine 9:1, 0 °C–rt; c 4,4′,4″-trimethoxytrityl chloride (1.8 eq.), pyridine, dimethylformamide (cat.), rt; d 1. 1 M NaOH (2.0 eq), MeOH, rt, 16 h; 2. 1,2-diaminoethane (7.0 eq), microwave, 140 °C, 16 h
The final deprotection of the amino and the carboxylic acid function of 7f, 14f, and 15f in order to obtain the free amino acids 7g, 14g, and 15g was first attempted in a two-step reaction sequence. Hydrazinolysis of the phthalimide moiety should liberate the terminal amino group, and subsequently hydrolysis of the methyl ester function under alkaline conditions (NaOH) the carboxylic acid moiety. Unfortunately, when the primary amine was formed in the first reaction step, it immediately reacted with the methyl ester function leading to the formation of the corresponding lactame, which could not be cleaved again without destruction of the molecule. Hence, the sequence of the deprotection was altered applying first NaOH to hydrolyse the methyl ester function. Thereby, also the phthaloyl group protecting the amino function was partially cleaved leading to the corresponding phthalamide moieties. Still, the free amino acids 7g, 14g, and 15g could be obtained by subjecting the thus obtained crude reaction product without prior isolation to heating with 1,2-diaminoethane. This furnished the desired target compounds 7g, 14g, and 15g in yields of 69–83% over both reaction steps (Scheme 6).
Biological evaluation
The amino acids 7a, 7b, 7g, 11d, 11g, 12g, 13d, 14a, 14b, 14g, 15a, 15b, 15g, as well as the carboxamide and ester derivatives 11c, 11f, 12f, and 13c were tested for their inhibitory potencies on the four GABA transporter subtypes mGAT1–4 in a [3H]GABA uptake assay previously developed by our group (Kragler et al. 2008) The tests were performed in a standardized manner in triplicates using HEK293 cell lines, each expressing one of the four GAT subtypes. In addition, binding affinities towards mGAT1 were examined employing a standardized MS Binding Assay with NO711 as native MS marker (Zepperitz et al. 2006). The results are summarized in Table 3. Inhibitory potencies and binding affinities of the tested compounds are represented as pIC50 and pKi, respectively. Each test compound was characterized in three independent experiments performed in triplicates and the standard error of mean (SEM) is given. If the determination of the pIC50 value proved not feasible due to low inhibitory potency, as percentage of the remaining [3H]GABA uptake at 100 µM concentration of the test compound. Correspondingly, the percentage of remaining MS marker is given in cases when the tested compound caused only a minor reduction of the MS marker binding.
As can be seen from the data in Table 3, the unsubstituted α- and β-hydroxyamino acids 7a, 14a, and 15a, which served as the starting material for the synthesis of the N-butyl derivatives 7b, 14b, and 15b, exhibit considerable inhibitory activities at most of the GAT subtypes, with the pIC50 values ranging from 4.06 ± 0.08 (15a, mGAT1, Table 3, entry 16) to 4.99 ± 0.08 (14a, mGAT1, Table 3, entry 13). Only for 7a at mGAT1 (pIC50 = 2.33 ± 0.05, Table 3, entry 2) and at mGAT2 (pIC50 = 3.38 ± 0.11, Table 3, entry 2) and 14a (pIC50 = 3.25 ± 0.02, Table 3, entry 13) at mGAT2 distinctly lower pIC50 values were found.
As compared with the unsubstituted α- and β-hydroxyamino acids 7a, 14a, and 15a the N-butyl derivatives 7b, 14b, and 15b are characterized by lower inhibitory activity throughout, which might indicate that the binding pose in which the amino nitrogen atom is oriented towards the cytosol, as postulated by molecular modelling experiments performed by Wein et al. (Wein et al. 2016) is energetically disfavoured. As reported in Table 3, entry 3, N-butylisoserine (7b) shows a pIC50 value of 3.26 ± 0.10 at mGAT4, which is on par with the value reported for racemic N-butylnipecotic acid 16 (pIC50 = 3.32 ± 0.04, Table 3, entry 1), which can be considered as a reasonable reference point since nipecotic acid constitutes the amino acid partial structure of many important GAT inhibitors including tiagabin (4, Table 1, entry 5) and (S)-SNAP-5114 (5, Table 1, entry 6). Interestingly, 7b exerts a somewhat higher inhibitory potency at mGAT3 (pIC50 = 3.54 ± 0.04) than at mGAT4, whereas the activity at mGAT1-2 can be considered negligible ([3H]GABA at 100 µM = 101% and 95%, respectively). This selectivity pattern deviates significantly from that of the reference compound 16, which exerts its highest inhibitory potency at mGAT1 (pIC50 = 4.11 ± 0.08) while being less active at mGAT2 (pIC50 = 3.23) and mGAT3 ([3H]GABA at 100 µM = 81%).
For the homologue of 7b, compound 14b, formally derived from 7b by inserting a methylene group at the end of the carboxyl acid carbon chain, the potency at mGAT4 has decreased distinctly, i.e. to a value below the identification threshold ([3H]GABA at 100 µM = 107%, Table 3, entry 14). The biological activity at mGAT3 on the other hand is only slightly reduced from 3.54 ± 0.04 for 7b to 3.21 ± 0.08 for 14b. The effects exerted at mGAT1 and mGAT2 are slightly higher as compared with 7b, with the remaining [3H]GABA uptake for 14b amounting to 79% (mGAT1) and 59% (mGAT2), respectively.
By contrast, if the elongation of the carbon chain is performed by inserting a methylene group between the carbon carrying the OH function and the carboxylic acid group (15b, Table 3, entry 17), the pIC50 value at mGAT4 increases nominally from 3.26 ± 0.10 for 7b to 3.42 ± 0.12 for 15b, while the effect exerted at mGAT3 remains unaltered (15b: pIC50 = 3.59 ± 0.07, 7b: pIC50 = 3.54 ± 0.04). As is the case with N-butylisoserine (7b), 15b exhibits no determinable inhibition at mGAT1 ([3H]GABA at 100 µM = 100%), whereas a slight effect at mGAT2 is found ([3H]GABA at 100 µM = 76%).
Unexpectedly, rigidization of the N-butylisoserine (7b) molecule by linking the C-2 atom with the nitrogen atom via a methylene bridge, resulting in azetidine heterocycle 11d, leads to a compound which exerts its highest inhibitory potency at mGAT2 (pIC50 = 3.38 ± 0.08, Table 3, entry 6). At the same time the effects of 11d at the other GATs are minor, the [3H]GABA uptake amounting to 72% (mGAT3), 85% (mGAT4), and 89% (mGAT1). Formal enlargement of the azetidine ring present in 11d to a piperidine ring causes the inhibitory potency at mGAT2 to completely vanish (13d, [3H]GABA at 100 µM = 104%, Table 3, entry 12). In addition, the activity exerted at the other GAT subtypes is extremely low, the values for the remaining [3H]GABA uptake at 100 µM being 96% (mGAT1), 85% (mGAT3), and 91% (mGAT4), respectively. As compared with its desoxy analogoue N-butylnipecotic acid (16, Table 3, entry 1), this constitutes a distinct decline of inhibitory potency at all GATs, hence demonstrating that the introduction of a hydroxy group into the 3-position of the nipecotic acid scaffold is associated with a strong reduction of biological activity.
11c and 13c, the carboxamide derivatives of the cyclic N-butyl-α-hydroxyamino acids 11d and 13d, were also tested for their inhibitory potential at all GAT subtypes. 11c, comprising an azetidine heterocyle, is characterized by marginal or non-detectable inhibitory potencies (Table 3, entry 5), the values of the remaining [3H]GABA uptake at 100 µM test compound concentration ranging from 83% (mGAT1) to nominally 105% (mGAT2). Likewise, 13c (Table 3, entry 11), which features a piperidine ring, reduces the [3H]GABA uptake to 84% (mGAT2), 79% (mGAT3) and 90% (mGAT4), whereas it appears to be completely inactive at mGAT1 at 100 µM, the [3H]GABA uptake amounting nominally to 109%.
Exhibiting pIC50 values of 3.59 ± 0.07 at mGAT3 and 3.42 ± 0.12 at mGAT4, 15b showed the highest inhibitory activity of the tested open-chain N-butylhydroxyamino acids (Table 3, entry 17). Hence, analogues of 15b that have the structure of the molecule rigidized by integrating the amino nitrogen atom and parts of the carbon chain into an azetidine (11g) and pyrrolidine ring (12g), respectively, seem of interest. However, 11g was found to exert only minor inhibitory potency at mGAT1-3, with the values for the remaining [3H]GABA uptake at 100 µM lying between 78% (mGAT2) and 80% (mGAT3) and the inhibitory potency at mGAT4 being negligible ([3H]GABA uptake at 100 µM = 99%, Table 3, entry 8). By contrast, the open-chain analogue 15b exerted reasonable inhibitory potencies at mGAT3 (pIC50 = 3.59 ± 0.07, Table 3, entry 17) and at mGAT4 (pIC50 = 3.42 ± 0.12).
Linking the C-3 atom and the amino nitrogen atom of 15b via a C2-bridge, resulting in a pyrrolidine substructure, is likewise accompanied by a complete loss of inhibitory potency at mGAT3-4, the values for the remaining [3H]GABA uptake being nominally 103% and 105%, respectively (12g, Table 3, entry 10). However, the compound exerts some activity at mGAT2 (pIC50 = 3.17). For mGAT1, a value of 94% remaining GABA uptake at 100 µM was determined, which is consistent with the parent compound 15b not showing any effect at this subtype (15b, remaining [3H]GABA uptake = 100%, Table 3, entry 17).
11f and 12f, the ester derivatives of the cyclic amino acids 11g and 12g, were also tested for their inhibitory potency at mGAT1–4. As shown in Table 3, entry 7, the azetidine derivative 11f exhibits some activity at mGAT1 (remaining [3H]GABA uptake = 66%), whereas the effect at mGAT2-4 is less pronounced (91%, 82% and 88%, respectively). 12f (Table 3, entry 9), which is the ester derivative of 12g, is characterized by minor or negligible inhibitory activity at all GAT subtypes, the values of the remaining [3H]GABA uptake being 104% (mGAT1), 96% (mGAT2), 86% (mGAT3) and 71% (mGAT4), respectively.
Since both the cyclic and acyclic N-butyl derivatives of 7a, 14a, and 15a were found to exhibit distinctly lower inhibitory potencies than the parent compounds consistently, derivatives of 7a, 14a, and 15a were synthesized which have the alcohol function linked to a C2 spacer bearing a 4,4′,4″-trimethoxytrityloxy moiety. In theory, this would allow the amino acid subunit to keep the more favourable orientation towards the intracellular space, while at the same time enabling interactions between the lipophilic domain and the extracellular vestibule, which are thought to significantly increase inhibitory potency and selectivity in case of nipecotic acid derivatives such as (S)-SNAP-5114 (5, Table 1, entry 6). Unfortunately, the introduction of the lipophilic domain into the scaffold of 7a, 14a, and 15a caused a reduction of inhibitory potency. For the derivatives resulting from this modification (7g, 14g, and 15g) the values for the [3H]GABA uptake at 100 µM test compound concentration remained at >50% at all transporter subtypes, equating to pIC50 values <4.0. Moreover, 7g and 15g were found to display only negligible subtype selectivity. In particular, 7g reduced the [3H]GABA uptake to 63% (mGAT1), 57% (mGAT2), 63% (mGAT3) and 60% (mGAT4), respectively (Table 3, entry 4). Likewise, the remaining [3H]GABA uptake in presence of 100 µM 15g ranged from 54% (mGAT3) to 67% (mGAT1 and mGAT2). For mGAT4, a pIC50 value just short below 4.0 was determined (pIC50 = 3.95, Table 3, entry 18). 14g (Table 3, entry 15) was found to reduce the [3H]GABA uptake to 78% (mGAT1), 66% (mGAT2), 94% (mGAT3) and 73% (mGAT4), respectively.
For all test substances the binding affinities at mGAT1 were found to be very low as compared with the reference compound 16 (pKi = 3.36 ± 0.02, Table 3, entry 1), the only exception being 14a (pKi = 3.61 ± 0.05, Table 3, entry 11).
Conclusion
A series of cyclic and acyclic hydroxyamino acid derivatives was synthesized and biologically evaluated for their potential as amino acid subunits in GAT inhibitors. Among the compounds synthesized for this study, we identified 1-butyl-3-hydroxyazetidine-3-carboxylic acid and, even more so, 2-(1-butyl-3-hydroxypyrrolidin-3-yl)acetic acid to be selective and moderately potent inhibitors of mGAT2, with the respective pIC50 values amounting to 3.17 and 3.38 ± 0.08, respectively.
Whereas the cyclic and hence more rigid N-butylhydroxyamino acids displayed only weak inhibitory potency at mGAT3 and mGAT4, moderate activity was observed in case of some acyclic compounds tested for this study. In particular, 4-(butylamino)-3-hydroxybutanoic acid (pIC50 = 3.42 ± 0.12) and N-butylisoserine (pIC50 = 3.26 ± 0.10) were found to be equal to N-butylnipecotic acid (pIC50 = 3.32) in terms of inhibitory potency at mGAT4, while being distinctly stronger mGAT3 inhibitors with the pIC50 values amounting to 3.59 ± 0.07 and 3.54 ± 0.04, respectively (N-butylnipecotic acid: [3H]GABA uptake at 100 µM = 81%). At the same time, both compounds exert no identifiable inhibitory effect at mGAT1 under the test conditions ([3H]GABA uptake at 100 µM = 100% and 101%, respectively), hence deviating strongly from the reference compound N-butylnipecotic acid, which constitutes a potent inhibitor of this GAT subtype (mGAT1: pIC50 = 4.11 ± 0.08). 4-(Butylamino)-3-hydroxybutanoic acid and N-butylisoserine might thus be suitable alternatives to the nipecotic acid subunit in mGAT3/4 inhibitors.
Interestingly, the pIC50 values of the unsubstituted, open-chained hydroxyamino acids are in most cases more than one log unit higher at all GAT subtypes than the values of the respective N-butyl analogues, indicating that N-derivatisation might carry an energy penalty by forcing the compound into a less favourable binding pose. Therefore, each of the hydroxyamino acids was derivatized by linking the alcohol function to a C2 spacer bearing a 4,4′,4″-trimethoxytrityloxy moiety, which is a common structural motif of mGAT4 inhibitors such as (S)-SNAP-5114. In theory, this would allow the amino acid subunit to adapt the more favourable binding pose, while at the same time enabling interactions between the target and the lipophilic domain. Unfortunately, the resulting compounds displayed poor inhibitory potency and selectivity as compared with the unsubstituted hydroxyamino acids and, even more so, as compared with (S)-SNAP-5114.
References
Bowery NG, Smart TG (2006) GABA and glycine as neurotransmitters: a brief history. Br J Pharmacol 147:S109–S119
Corey JL, Guastella J, Davidson N, Lester HA (1994) GABA uptake and release by a mammalian cell line stably expressing a cloned rat brain GABA transporter. Mol Membr Biol 11:23–30
Daemen MA, Hoogland G, Cijntje JM, Spincemaille GH (2008) Upregulation of the GABA transporter GAT-1 in the spinal cord contributes to pain behaviour in experimental neuropathy. Neurosci Lett 444:112–115
Dhar TGM, Borden LA, Tyagarajan S, Smith KE, Branchek TA, Weinshank RL, Gluchowski C (1994) Design, synthesis and evaluation of substituted triarylnipecotic acid derivatives as GABA uptake inhibitors: identification of a ligand with moderate affinity and selectivity for the cloned human GABA transporter GAT-3. J Med Chem 37:2334–2342
Farkas M, Li B, Dose C, Dervan P (2009) DNA sequence selectivity of hairpin polyamide turn units. Bioorg Med Chem Lett 19:3919–3923
Jin XT, Galvan A, Wichmann T, Smith Y (2011) Localization and function of GABA transporters GAT-1 and GAT-3 in the basal ganglia. Front Syst Neurosci 5:63
Kalueff AV, Nutt DJ (2007) Role of GABA in anxiety and depression. Depress Anxiety 24:495–517
Kragler A, Höfner G, Wanner KT (2005) Novel parent structures for inhibitors of the murine GABA transporters mGAT3 and mGAT4. Eur J Pharmacol 519:43–47
Kragler A, Höfner G, Wanner KT (2008) Synthesis of aminomehtylphenol derivatives as inhibitors of the murine GABA transporters mGAT1-mGAT4. Eur J Med Chem 43:2404–2411
Kristensen AS, Andersen J, Jørgensen TN, Sørensen L, Eriksen J, Loland CJ, Strømgaard K, Gether U (2011) SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol Rev 63:585–640
Krogsgaard-Larsen P, Falch E, Larsson OM, Schousboe A (1991) GABA uptake inhibitors: kinetics and molecular pharmacology. Adv Biosci 82:197–200
Krogsgaard-Larsen P, Frolund B, Frydenvang K (2000) GABA uptake inhibitors: design, molecular pharmacology and therapeutic aspects. Curr Pharm Des 6:1193–1209
Lamb P (2008) Methods of using combinations of MEK and JAK-2 inhibitors. PCT Int Appl 2008:124085
Lanctôt KL, Herrmann N, Mazzotta P, Khan LR, Ingber N (2004) GABAergic function in Alzheimer's disease: evidence for dysfunction and potential as a therapeutic target for the treatment of behavioural and psychological symptoms of dementia Can J Psychiatry 49(7):439–453
Madsen KK, Clausen RP, Larsson OM, Krogsgaard-Larsen P, Schousboe A, White HS (2009) Synaptic and extrasynaptic GABA transporters as targets for anti-epileptic drugs. J Neurochem 109:139–144
Meldrum BS, Chapman AG (1999) Basic mechanisms of gabitril (tiagabine) and future potential developments. Epilepsia 40:S2–S6
Minelli A, DeBiasi S, Brecha NC, Zuccarello LV, Conti F (1996) GAT-3, a high-affinity GABA plasma membrane transporter, is localized to astrocytic processes, and it is not confined to the vicinity of GABAergic synapses in the cerebral cortex. J Neurosci 16:6255
Nielsen EB, Suzdak PD, Andersen KE, Knutsen LJ, Sonnewald U, Braestrup C (1991) Characterization of tiagabine (NO-328), a new potent and selective GABA uptake inhibitor. Eur J Pharmacol 196:257–266
Santimukul S, Perez JM (2011) Selective N-Alkylation of β-alanine facilitates the synthesis of a Poly(amino acid)-based theranostic nanoagent. Biomacromolecules 12:3917–3927
Seth A, Sharma PA, Tripathi A, Choubey PK, Srivastava P, Tripathi PN, Shrivastava SK (2018) Design, synthesis, evaluation and computational studies of nipecotic acid-acetonaphthone hybrids as potential antiepileptic agents. Med Chem 14:409–426
Steffan T, Renukappa-Gutke T, Höfner G, Wanner KT (2015) Design, synthesis and SAR studies of GABA uptake inhibitors derived from 2-substituted pyrrolidine-2-yl-acetic acids. Bioorg Med Chem 23:1284–1306
Treiman DM (2001) GABAergic mechanisms in epilepsy. Epilepsia 42:8–12
Wein T, Petrera M, Allmendinger L, Höfner G, Pabel J, Wanner KT (2016) Different binding modes of small and large binders of GAT1. Chem Med Chem 11:509–518
White HS, Sarup A, Bolvig T, Kristensen A, Petersen G, Nelson N, Pickering D, Larsson OM, Frølund B, Krogsgaard-Larsen P, Schousboe A (2002) Correlation between anticonvulsant activity and inhibitory action on glial gamma-aminobutyric acid uptake of the highly selective mouse gamma-aminobutyric acid transporter 1 inhibitor 3-hydroxy-4-amino-4,5,6,7-tetrahydro-1,2-benzisoxazole and its N-alkylated analogues. J Pharmacol Exp Ther 302:636–644
Zepperitz C, Höfner G, Wanner KT (2006) MS-Binding assays: kinetic, saturation and competitive experiments based on quantitation of bound marker—exemplified by the GABA transporter mGAT1. ChemMedChem 1:208–217
Zhou Y, Holmseth S, Hua R, Lehre AC, Olofsson AM, Poblete-Naredo I, Kempson SA, Danbolt NC (2012) The betaine-GABA transporter (BGT1, slc6a12) is predominantly expressed in the liver and at lower levels in the kidneys and at the brain surface. Am J Physiol Ren Physiol 302:F316–F328
Acknowledgements
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Andreß, J.C., Böck, M.C., Höfner, G. et al. Synthesis and biological evaluation of α- and β-hydroxy substituted amino acid derivatives as potential mGAT1–4 inhibitors. Med Chem Res 29, 1321–1340 (2020). https://doi.org/10.1007/s00044-020-02548-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-020-02548-x