Abstract
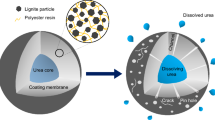
Biodegradable coating materials based on PCL grafted on guar gum and halloysite nanotubes have been synthetized and used to delay the nutrients release from Diammonium Phosphate (DAP) as water-soluble fertilizer granules. The combination of hydrophobic character of PCL as biodegradable polymer and the swelling behavior of guar gum (GG) and halloysite nanotubes (HNT) as natural fillers were evaluated in this study with a view of the slow release of fertilizers. Indeed, the hydroxyl groups on the guar gum (GG) and halloysite (HNTs) surfaces act as initiators for caprolactone in situ ring opening polymerization and the developing polymers are covalently grafted to the GG and HNTs. Moreover, the hydrophilic character of fillers (GG & HNT) improves the adhesion between the coating agent and the surface of the (DAP) granules and consequently DAP granules were successfully coated by the dip-coating process with fixed thickness of ~ 25 µm as revealed by the scanning electron microscopy. The evaluation of the release behavior of DAP coated by the composites (PCL-g-GG and PCL-g-HNT) demonstrates that the liberation rate could be controlled by adjusting the fillers (GG or HNT) contents in the coating materials (PCL-g-GG and PCL-g-HNT). This encapsulation expands the nitrogen and phosphorus release to more than 50 h instead of only 2 h for uncoated DAP granules.









Similar content being viewed by others
References
Brown ME, Higgins N (2009) Markets, climate change, and food security in West Africa. Environ Sci Technol 43:8016–8020
FAO (2009) Global agriculture towards 2050. In: High lev expert forum-how to feed world 2050, pp 1–4
Trenkel ME (2010) Slow- and controlled-release and stabilized fertilizers: an option for enhancing nutrient use efficiency in agriculture
Rindt DW, Blouin GM, Getsinger JG (1968) Sulfur coating on nitrogen fertilizer to reduce dissolution rate. J Agric Food Chem 16:773–778
Blouin GM, Rindt DW, Moore OE (1971) Sulfur-coated fertilizers for controlled release: pilot plant production. J Agric Food Chem 19:801–808
Al-Zahrani SM (2000) Utilization of polyethylene and paraffin waxes as controlled delivery systems for different fertilizers. Ind Eng Chem Res 39:367–371
Entry JA, Sojka RE (2008) Matrix based fertilizers reduce nitrogen and phosphorus leaching in three soils. J Environ Manag 87:364–372
Wu L, Liu M, Liang Rui (2008) Preparation and properties of a double-coated slow-release NPK compound fertilizer with superabsorbent and water-retention. Bioresour Technol 99:547–554
Yu JH, Wang JL, Wu X et al (2016) Utilization of starch films plasticized with urea as fertilizer for improvement of plant growth. Starch/Staerke 40:1–8
Wei X, Bao X, Yu L et al (2020) Correlation between gel strength of starch-based hydrogel and slow release behavior of its embedded urea. J Polym Environ
Li T, Gao B, Tong Z et al (2019) Chitosan and graphene oxide nanocomposites as coatings for controlled-release fertilizer. Water Air Soil Pollut 230:246
Ko B, Cho Y, Rhee H (1996) Controlled release of urea from rosin-coated fertilizer particles. Ind Eng Chem Res 35(1):250–257
Han Q, Shao B, Guo CG, Liu YX (2010) Synthesis and characterization of carboxymethylcellulose and methyl methacrylate graft copolymers. Pigment Resin Technol 3:156–162
Ilsouk M, Raihane M, Lahcini M et al (2017) Organomodified Moroccan beidellite clay prepared by in situ ring opening polymerization: characterizations and properties. J Macromol Sci Part A 54:201–210
Boujemaoui A, Carlsson L, Malmstro E et al (2012) Facile preparation route for nanostructured composites: surface- initiated ring-opening polymerization of ε -caprolactone from high- surface-area nanopaper. ACS Appl Mater Interfaces 4:3191–3198
Öchsner A, Shokuhfar A (2013) New frontiers of nanoparticles and nanocomposite materials: novel principles and techniques. Springer, Berlin
Liang R, Liu M (2006) Preparation and properties of a double-coated slow-release and water-retention urea fertilizer. J Agric Food Chem 54:1392–1398
Wu L, Liu M (2008) Preparation and properties of chitosan-coated NPK compound fertilizer with controlled-release and water-retention. Carbohydr Polym 72:240–247
Ni B, Liu M, Lü S (2009) Multifunctional slow-release urea fertilizer from ethylcellulose and superabsorbent coated formulations. Chem Eng J 155:892–898
Lahcini M, Elhakioui S, Szopinski D et al (2016) Harnessing synergies in tin-clay catalyst for the preparation of poly (e -caprolactone)/halloysite nanocomposites. Eur Polym J 81:1–11
El Assimi T, Blažic R, El Kadib A et al (2019) Synthesis of poly(ε-caprolactone)-grafted guar gum by surface-initiated ring-opening polymerization. Carbohydr Polym 220:95–102
Guo Y, Wang X, Shen Z et al (2013) Preparation of cellulose-graft-poly(ε-caprolactone) nanomicelles by homogeneous ROP in ionic liquid. Carbohydr Polym 92:77–83
Mark JE (1999) Polymer data handbook, J.E. Mark. Oxford University Press, New York
Chum ZZ, Woodruff MA, Cool SM, Hutmacher DW (2009) Porcine bone marrow stromal cell differentiation on heparin-adsorbed poly(e-caprolactone)-tricalcium phosphate-collagen scaffolds. Acta Biomater 5:3305–3315
Van De Velde K, Kiekens P (2002) Biopolymers: overview of several properties and consequences on their applications. Polym Test 21:433–442
Labet M, Thielemans W (2009) Synthesis of polycaprolactone: a review. Chem Soc Rev 38:3484
Devassine M, Henry F, Guerin P, Briand X (2002) Coating of fertilizers by degradable polymers. Int J Pharm 242:399–404
Wu KJ, Wu CS, Chang JS (2007) Biodegradability and mechanical properties of polycaprolactone composites encapsulating phosphate-solubilizing bacterium Bacillus sp. PG01. Process Biochem 42:669–675
Lönnberg H, Zhou Q, Brumer H et al (2006) Grafting of cellulose fibers with poly(ε-caprolactone) and poly(L-lactic acid) via ring-opening polymerization. Biomacromol 7:2178–2185
Wu CS (2004) Analysis of mechanical, thermal, and morphological behavior of polycaprolactone/wood flour blends. J Appl Polym Sci 94:1000–1006
Wu C (2003) Physical properties and biodegradability of maleated-polycaprolactone/starch composite. Polym Degrad Stab 80:127–134
Thombare N, Jha U, Mishra S, Siddiqui MZ (2016) International journal of biological macromolecules guar gum as a promising starting material for diverse applications: a review. Int J Biol Macromol 88:361–372
Seeli DS, Prabaharan M (2016) International journal of biological macromolecules guar gum succinate as a carrier for colon-specific drug delivery. Int J Biol Macromol 84:10–15
Sharma R, Kaith BS, Kalia S et al (2015) Biodegradable and conducting hydrogels based on Guar gum polysaccharide for antibacterial and dye removal applications. J Environ Manag 162:37–45
Szopinski D, Kulicke W, Luinstra GA (2015) Structure—property relationships of carboxymethyl hydroxypropyl guar gum in water and a hyperentanglement parameter. Carbohydr Polym 119:159–166
Silberbush M, Adar E (1993) Use of an hydrophilic polymer to improve water storage and availability to crops grown in sand dunes I. Corn irrigated by trickling. Agric Water Manag 23:303–313
Thombare N, Mishra S, Siddiqui MZ et al (2018) Design and development of guar gum based novel, superabsorbent and moisture retaining hydrogels for agricultural applications. Carbohydr Polym 185:169–178
Du M, Jia D (2010) Newly emerging applications of halloysite nanotubes: a review. Polym Int 59:574–582
Enyashin AN, Seifert G, Duarte A (2010) Structural, electronic, and mechanical properties of single-walled halloysite nanotube models. J Phys Chem C 114:11358–11363
Xie Y, Chang PR, Wang S et al (2011) Preparation and properties of halloysite nanotubes/plasticized Dioscorea opposita Thunb. Starch composites. Carbohydr Polym 83:186–191
Lam CW, James JT, McCluskey R et al (2006) A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit Rev Toxicol 36:189–217
Korsmyer RW, Gumy R, Doelker E et al (1983) Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm 15:25–35
Costa MME, Cabral-Albuquerque ECM, Alves TLM et al (2013) Use of polyhydroxybutyrate and ethyl cellulose for coating of urea granules. J Agric Food Chem 61:9984–9991
Li J, Wang M, She D, Zhao Y (2017) Structural functionalization of industrial softwood kraft lignin for simple dip-coating of urea as highly efficient nitrogen fertilizer. Ind Crops Prod 109:255–265
Tomaszewska M, Jarosiewicz A (2006) Encapsulation of mineral fertilizer by polysulfone using a spraying method. Desalination 198:346–352
Ritger PL, Peppas NA (1987) A simple equation for description of solute release II. Fickien and anomalus release from swellable devices. J Control Release 5:37–42
Ritger PL, Peppas NA (1987) A simple equation for description of solute release I. Fickien and non-fickien rlease from swellable devices in the form slabs, spheres, cylinders pr discs. J Control Release 5:23–36
Lahcini M, Castro PM, Kalmi M, Leskela M (2004) The use of tetra (phenylethynyl) tin as an initiator for the ring-opening polymerization of lactide. Organometallics 23:4547–4549
De Silva RT, Pasbakhsh P, Goh KL et al (2013) Physico-chemical characterisation of chitosan/halloysite composite membranes. Polym Test 32:265–271
Olad A, Zebhi H, Salari D et al (2018) Materials Science & Engineering C Slow-release NPK fertilizer encapsulated by carboxymethyl cellulose-based nanocomposite with the function of water retention in soil. Mater Sci Eng, C 90:333–340
Jamnongkan T, Kaewpirom S (2010) Controlled-release fertilizer based on chitosan hydrogel: phosphorus release kinetics. Sci J 1:43–50
Place ES, George JH, Williams CK, Stevens MM (2009) Synthetic polymer scaffolds for tissue engineering. Chem Soc Rev 38:1139–1151
Castilla-Cortázar I, Más-Estellés J, Meseguer-Dueñas JM et al (2012) Hydrolytic and enzymatic degradation of a poly(ε-caprolactone) network. Polym Degrad Stab 97:1241–1248
Li S (1998) Hydrolytic degradation characteristics of aliphatic polyesters derived from lactic and glycolic acids. J Biomed Mater Res 48:342–353
Xiu JQ, Yuan CL, Dong HX et al (2017) Behavior of ammonium adsorption by clay mineral halloysite. Trans Nonferr Met Soc China 27:1627–1635
Saki H, Alemayehu E, Schomburg J, Lennartz B (2019) Halloysite nanotubes as adsorptive material for phosphate removal from aqueous solution. Water 11:203
Zheng Y, Wang A (2010) Enhanced adsorption of ammonium using hydrogel composites based on chitosan and halloysite. J Macromol Sci Part A 47:33–38
Acknowledgements
The Authors would like to acknowledge the support through the R&D Initiative—Appel à projets autour des phosphates APPHOS—sponsored by OCP (OCP Foundation, R&D OCP, Mohammed VI Polytechnic University, National Centre of Scientific and technical Research CNRST, Ministry of Higher Education, Scientific Research and Professional Training of Morocco MESRSFC) under the project entitled “Stabilisation et liberation contrôlée des fertilisants par enrobage des engraisphosphatés par de nouvelles formulations polymères: uneoption de l’utilisationefficace des nutriments dans l’agriculture”, Project IDVAL-RAI-01/2017 and the CNRST-Morocco, Projet Prioritaire PPR1/2015/73 and Alexander von Humboldt Foundation for supporting synthesis of bio-nanocomposites.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
El Assimi, T., Chaib, M., Raihane, M. et al. Poly(ε-caprolactone)-g-Guar Gum and Poly(ε-caprolactone)-g-Halloysite Nanotubes as Coatings for Slow-Release DAP Fertilizer. J Polym Environ 28, 2078–2090 (2020). https://doi.org/10.1007/s10924-020-01750-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-020-01750-7