Abstract
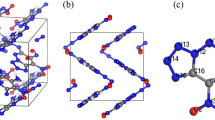
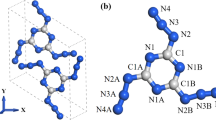
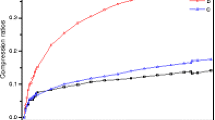
In order to find out the relationship between external pressures and properties of energetic materials, we used the density functional theory (DFT) method to investigate the structural, electronic, and absorption properties of crystalline 2,4,6-trinitrotoluene (TNT)/2,4,6-trinitrotoluene (TNB) under hydrostatic compression of 0–100 GPa. By analyzing the change of lattice constants (a, b, and c) of TNT/TNB under compression conditions, we found that variation tendency of the lattice constants was anisotropic. The b-axis is much stiffer than that along the a- and c-axes, which indicates that the TNT/TNB crystal is anisotropic within a certain pressure region. The pressure-induced structure transformation results in the new covalent bonds O11-C13, O12-C11, O8-C4, and O1-C12 at 60 GPa, and O4-C5 at 80 GPa, respectively. By analyzing the band structure and density of states of TNT/TNB in the pressure range over 40 GPa, the electronic structure of TNT/TNB changed to metallic system, which indicated it becomes more sensitivity under high pressures. The pressure-induced structure transformation of TNT/TNB also contributed to the relatively high optical activity of TNT/TNB at 70 GPa.













Similar content being viewed by others
Data availability
The source of data and materials were mentioned in the manuscript.
References
Desiraju GR (2013) Crystal engineering: from molecule to crystal. J Am Chem Soc 135(27):9952–9967. https://doi.org/10.1021/ja403264c
Bolton O, Matzger AJ (2011) Improved stability and smart-material functionality realized in an energetic cocrystal. Angew Chem Int Ed 50(38):8960–8963. https://doi.org/10.1002/anie.201104164
Jiao F, Xiong Y, Li H, Zhang C (2018) Alleviating the energy & safety contradiction to construct new low sensitivity and highly energetic materials through crystal engineering. CrystEngComm 20(13):1757–1768. https://doi.org/10.1039/C7CE01993A
Tian B, Xiong Y, Chen L, Zhang C (2018) Relationship between the crystal packing and impact sensitivity of energetic materials. CrystEngComm 20(6):837–848. https://doi.org/10.1039/C7CE01914A
Zhang C, Wang X, Huang H (2008) π-stacked interactions in explosive crystals: buffers against external mechanical stimuli. J Am Chem Soc 130(26):8359–8365. https://doi.org/10.1021/ja800712e
Yang Z, Li H, Zhou X, Zhang C, Huang H, Li J, Nie F (2012) Characterization and properties of a novel energetic–energetic cocrystal explosive composed of HNIW and BTF. Cryst Growth Des 12(11):5155–5158. https://doi.org/10.1021/cg300955q
Bolton O, Simke LR, Pagoria PF, Matzger AJ (2012) High power explosive with good sensitivity: a 2: 1 cocrystal of CL-20: HMX. Cryst Growth Des 12(9):4311–4314. https://doi.org/10.1021/cg3010882
Yang Z, Wang Y, Zhou J, Li H, Huang H, Nie F (2014) Preparation and performance of a BTF/DNB cocrystal explosive. Propellants, Explos, Pyrotech 39(1):9–13. https://doi.org/10.1002/prep.201300086|
Guo C, Zhang H, Wang X, Liu X, Sun J (2013) Study on a novel energetic cocrystal of TNT/TNB. J Mater Sci 48(3):1351–1357. https://doi.org/10.1007/s10853-012-6881-5
Shokrolahi A, Zali A, Mousaviazar A et al (2011) Preparation of nano-K-6 (nano-Keto RDX) and determination of its characterization and thermolysis. J Energ Mater 29(2):115–126. https://doi.org/10.1080/07370652.2010.507237
Zhang M, Gao H, Li C et al (2017) Towards improved explosives with a high performance: N-(3, 5-dinitro-1 H-pyrazol-4-yl)-1 H-tetrazol-5-amine and its salts. J Mater Chem A 5(4):1769–1777. https://doi.org/10.1039/C6TA07740D
Yin P, Zhang Q, Shreeve JM (2015) Dancing with energetic nitrogen atoms: versatile N-functionalization strategies for N-heterocyclic frameworks in high energy density materials. Acc Chem Res 49(1):4–16. https://doi.org/10.1021/acs.accounts.5b00477
Golovina NI, Goncharov TK, Dubikhin VV et al (2009) Kinetics and mechanism of the thermal decomposition of keto-RDX. Russ J Phys Chem B 3(6):896–900. https://doi.org/10.1134/S1990793109060062
Özhan G, Topuz S, Alpertunga B (2003) Determination of cyclonite (RDX) in human plasma by high-performance liquid chromatography. Il Farmaco 58(6):445–448. https://doi.org/10.1016/S0014-827X(03)00069-7
Gholamian F, Ansari M, Abdullah M et al (2013) Intermolecular interaction of a neutral polymeric bonding agent containing N-Vinylpyrrolidone units with ammonium perchlorate and Keto-RDX. Chin J Polym Sci 31(10):1372–1381. https://doi.org/10.1007/s10118-013-1327-3
Pulham CR, Millar D I A, Oswald IDH, et al (2010) High-pressure studies of energetic materials. High-Pressure Crystallography. Springer, Dordrecht, pp 447–457
Fabbiani FPA, Pulham CR (2006) High-pressure studies of pharmaceutical compounds and energetic materials[J]. Chem Soc Rev 35(10):932–942. https://doi.org/10.1039/B517780B
Ma P, Pan Y, Jiang J et al (2017) A novel energetic perchlorate amine salt: synthesis, properties, and density functional theory calculation. J Energ Mater 35(4):443–457. https://doi.org/10.1080/07370652.2016.1269851
Ma P, Jiang JC, Zhu SG (2017) Synthesis, XRD and DFT studies of a novel cocrystal energetic perchlorate amine salt: methylamine triethylenediamine triperchlorate. Combust Explos Shock Waves 53(3):319–328. https://doi.org/10.1134/S0010508217030091
Ma P, Pan Y, Jiang J et al (2018) Molecular dynamic simulation and density functional theory insight into the nitrogen rich explosive 1, 5-diaminotetrazole (DAT). Proc Eng 211:546–554. https://doi.org/10.1016/j.proeng.2017.12.047
Hu A, Larade B, Dudiy S et al (2007) Theoretical prediction of heats of sublimation of energetic materials using pseudo-atomic orbital density functional theory calculation. Propellants, Explos, Pyrotech 32(4):331–337. https://doi.org/10.1002/prep.200700037
Ma P, Jin YT, Wu PH et al (2017) Synthesis, molecular dynamic simulation, and density functional theory insight into the cocrystal explosive of 2, 4, 6-trinitrotoluene/1, 3, 5-trinitrobenzene. Combust Explos Shock Waves 53(5):596–604. https://doi.org/10.1134/S0010508217050148
Wei H, He C, Zhang J et al (2015) Combination of 1, 2, 4-oxadiazole and 1, 2, 5-oxadiazole moieties for the generation of high-performance energetic materials. Angew Chem Int Ed 54(32):9367–9371. https://doi.org/10.1002/anie.201503532|
Segall MD, Lindan PJD, Probert MJ et al (2002) First-principles simulation: ideas, illustrations and the CASTEP code. J Phys 14(11):2717–2744. https://doi.org/10.1088/0953-8984/14/11/301
Fischer TH, Almlof J (1992) General methods for geometry and wave function optimization. J Phys Chem 96:976. https://doi.org/10.1021/j100203a036
Budzevich MM, Landerville AC, Conroy MW et al (2010) Hydrostatic and uniaxial compression studies of 1, 3, 5-triamino-2, 4, 6-trinitrobenzene using density functional theory with van der Waals correction[J]. J Appl Phys 107(11):113524. https://doi.org/10.1063/1.3361407
Zhu WH, Xiao JJ, Ji GF, Zhao F, Xiao HM (2007) First-principles study of the four polymorphs of crystalline octahydro-1,3,5,7-tetranitro- 1,3,5,7-tetrazocine. J PhysChem B 111:12715–12722. https://doi.org/10.1021/jp075056v
Kuklja MM, Stefanovich EV, Kunz AB (2000) An excitonic mechanism of detonation initiation in explosives. J Chem Phys 12:3417–3423. https://doi.org/10.1063/1.480922
Ma P, Wang J, Zhai D, Hao L, Ma C, Pan Y, Zhu S (2019) Structural transformation and absorption properties of 2, 4, 6-Trinitro-2, 4, 6-triazacyclohexanone under high pressures. J Mol Struct 1196:691–698. https://doi.org/10.1016/j.molstruc.2019.07.020
Wu Q, Zhu WH, Xiao HM (2013) First-principles study of the four polymorphs of crystalline octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine. J Phys Chem C 117:16830–16839. https://doi.org/10.1021/jp075056v
Ma Q, Huang SL, Lu HC, Nie F, Liao LY, Fan GJ, Huang JL (2019) Energetic cocrystal, ionic salt, and coordination polymer of a perchlorate free high energy density oxidizer: influence of p K a modulation on their formation. Cryst Growth Des 19(2):714–723. https://doi.org/10.1021/acs.cgd.8b01293
Şen N (2019) Characterization and properties of a new energetic co-crystal composed of trinitrotoluene and 2, 6-diaminotoluene. J Mol Struct 1179:453–461. https://doi.org/10.1016/j.molstruc.2018.11.013
Zhu W, Xiao H (2010) First-principles band gap criterion for impact sensitivity of energetic crystals: a review. Struct Chem 657–665. https://doi.org/10.1007/s11224-010-9596-8
Saha S, Sinha TP, Mookerjee A (2000) Electronic structure, chemical bonding, and optical properties of paraelectric BaTiO 3. Phys Rev B 62(13):8828. https://doi.org/10.1103/PhysRevB.62.8828
Acknowledgments
We are grateful to the High Performance Computing Center of Nanjing Tech University for supporting the computational resources.
Funding
This work is supported by the National Natural Science Foundation of China (Grant No. 11702129).
Author information
Authors and Affiliations
Contributions
Peng Ma designed and performed experiments and wrote the paper; Lina Hao and Xuqin Liu designed the experiments; Diandian Zhai and Jinpeng Wang performed the experiments; Congming Ma, Yong Pan, and Juncheng Jiang supervised the project; Lin Zhang and Shunguan Zhu analyzed the data.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, P., Liu, X., Hao, L. et al. Pressure induced structural behavior of energetic cocrystal TNT/TNB: a density functional theory study. J Mol Model 26, 121 (2020). https://doi.org/10.1007/s00894-020-04394-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04394-5