Abstract
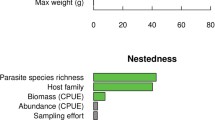
The extinction of fish species can direct and indirectly affect many groups of associated species, among which parasite communities can be the most susceptible. However, the intensity of this effect depends on the structure interaction networks. This study evaluated whether networks constituted of fish ectoparasites or endoparasites differed in their robustness to the loss of host species and to what extent these potential differences are explained by the network structures. We used path models to evaluate the direct and indirect effects of host and parasite richness, connectance, and nestedness on the robustness of ecto- and endoparasite-based networks. In most cases, nestedness was the descriptor that best explained the robustness of the fish-parasite networks, and co-extinctions are less likely when the fish species act mainly as hosts of the generalist parasites. Both the richness of the host species and connectance in the networks have an essential indirect influence on robustness. Regardless of the extinction sequence, the ectoparasite-based networks showed higher vulnerability to host species loss when compared to endoparasite-based networks. These findings highlight the importance of considering both ecto- from endoparasites to better understand the structure and vulnerability of host–parasite networks.


Similar content being viewed by others
References
Almeida-Neto, M. & W. Ulrich, 2011. A straightforward computational approach for measuring nestedness using quantitative matrices. Environmental Modelling & Software 26(2): 173–178.
Almeida-Neto, M., P. Guimarães, P. R. Guimarães, R. D. Loyola & W. Ulrich, 2008. A consistent metric for nestedness analysis in ecological systems: reconciling concept and measurement. Oikos 117(8): 1227–1239.
Arbuckle, J. L., 2003. AMOS (Version 5.0). Small Waters Corporation, Chicago.
Bellay, S., E. F. de Oliveira, M. Almeida-Neto, D. P. Lima-Jr, R. M. Takemoto & J. L. Luque, 2013. Developmental stage of parasites influences the structure of fish-parasite networks. PLoS ONE 8(10): e75710.
Bellay, S., E. F. de Oliveira, M. Almeida-Neto, M. A. R. Mello, R. M. Takemoto & J. L. Luque, 2015. Ectoparasites and endoparasites of fish form networks with different structures. Parasitology 142(7): 901–909.
Bellay, S., F. H. Oda, K. M. Campião, F. H. Yamada, R. M. Takemoto & E. F. de Oliveira, 2018. Host-parasite networks: an integrative overview with tropical examples. In Dáttilo, W. & V. Rico-Gray (eds), Ecological Networks in the Tropics. Springer, Cham: 127–140. https://doi.org/10.1007/978-3-319-68228-0_9.
Bersier, L. F., P. Dixon & G. Sugihara, 1999. Scale-invariant or scale-dependent behavior of the link density property in food webs: a matter of sampling effort? American Naturalist 153(6):676–682.
Burgos, E., H. Ceva, R. P. J. Perazzo, M. Devoto, D. Medan, M. Zimmermann & A. María Delbue, 2007. Why nestedness in mutualistic networks? Journal of Theoretical Biology 249(2): 307–313.
Bush, A. O., J. C. Fernández, G. W. Esch & J. R. Seed (eds), 2001. Parasitism: The Diversity and Ecology of Animal Parasites. Cambridge University Press, Cambridge: 566 pp.
Costello, C., D. Ovando, T. Clavelle, C. K. Strauss, R. Hilborn, M. C. Melnychuk, T. A. Branchc, S. D. Gainesa, C. S. Szuwalskia, R. B. Cabrala, D. N. Raderb & A. Leland, 2016. Global fishery prospects under contrasting management regimes. Proceedings of the National Academy of Sciences of the United States of America 113(18): 5125–5129.
Dallas, T. & E. Cornelius, 2015. Co-extinction in a host–parasite network: identifying key hosts for network stability. Scientific Reports 5: 13185.
Darwall, W. R. T. & J. Freyhof, 2016. Lost fishes, who is counting? The extent of the threat to freshwater fish biodiversity. In G. P. Closs, M. Krkosek & J. D. Olden (eds), Conservation of Freshwater Fishes. Cambridge University Press, Cambridge.
Dormann, C. F., B. Gruber & J. Fründ, 2008. Introducing the bipartite Package: analysing ecological networks. R News 8: 8–11.
Dunne, J. A., R. J. Williams & N. D. Martinez, 2002. Network structure and biodiversity loss in food webs: robustness increases with connectance. Ecology Letters 5(4): 558–567.
Eiras, J., H. Segner, T. Wahli & B. G. Kapoor (eds), 2008. Fish Diseases, 2 vols. CRC Press, Boca Raton: 1340 pp.
Farrell, M. J., P. R. Stephens, L. Berrang-Ford, J. L. Gittleman & T. J. Davies, 2015. The path to host extinction can lead to loss of generalist parasites. Journal of Animal Ecology 84: 978–984.
Frainer, A., B. G. McKie, P. Amundsen, R. Knudsen & K. D. Lafferty, 2018. Parasitism and the biodiversity–functioning relationship. Trends in Ecology and Evolution 33(4):260-268.
Franco, J. L. de A., 2013. The concept of biodiversity and the history of conservation biology: from wilderness preservation to biodiversity conservation. História (São Paulo) 32(2): 21–48.
Gilbert, A. J., 2009. Connectance indicates the robustness of food webs when subjected to species loss. Ecological Indicators 9(1): 72–80.
Hatcher, M. J., J. T. Dick & A. M. Dunn, 2012. Diverse effects of parasites in ecosystems: linking interdependent processes. Frontiers in Ecology and the Environment 10(4): 186–194.
Kolbert, E., 2014. The Sixth Extinction: An Unnatural History. Henry Holt and Company, New York: 319 pp.
Lima, L. B., S. Bellay, H. C. Giacomini, A. Isaac & D. P. Lima-Jr, 2016. Influence of host diet and phylogeny on parasite sharing by fish in a diverse tropical floodplain. Parasitology 143(3): 343–349.
Lima-Jr, D. P., H. C. Giacomini, R. M. Takemoto, A. A. Agostinho & L. M. Bini, 2012. Patterns of interactions of a large fish–parasite network in a tropical floodplain. Journal of Animal Ecology 81(4): 905–913.
MacCallum, R. C., M. W. Browne & H. M. Sugawara, 1996. Power analysis and determination of sample size for covariance structure modeling. Psychological Methods 1(2): 130–149.
Martinez, N. D., B. A. Hawkins, H. A. Dawah & B. P. Feifarek, 1999. Effects of sampling effort on characterization of food-web structure. Ecology 80(3): 1044–1055.
Memmott, J., N. W. Waser & M. V. G. Price, 2004. Tolerance of pollinator networks to species extinctions. Proceedings of the Royal Society B 271(1557): 2605–2611.
Olesen, J. M., J. Bascompte, Y. L. Dupont & P. Jordano, 2007. The modularity of pollination networks. Proceedings of the National Academy of Sciences of the United States of America 104: 19891–19896.
Pascual, M. & J. A. Dunne (eds), 2006. Ecological Networks: Linking Structure to Dynamics in Food Webs. Oxford University Press, New York: 386 pp.
Pimm, S. L., 1982. Food Webs. Chapman & Hall, London: 219 pp.
Poulin, R., 2014. Parasite biodiversity revisited: frontiers and constraints. International Journal for Parasitology 44(9): 581–589.
Preacher, K. J. & D. L. Coffman, 2006. Computing power and minimum sample size for RMSEA. https://quantpsy.org/.
R Development Core Team, 2019. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna.
Rohde, K. & M. Keap, 1998. Latitudinal differences in species and community richness and in community structure of metazoan endo- and ectoparasites of marine teleost fish. International Journal of Parasitology 28: 461–474.
Rynkiewicz, E. C., A. B. Pedersen & A. Fenton, 2015. An ecosystem approach to understanding and managing within-host parasite community dynamics. Trends in Parasitology 31: 212–221.
Santamaria, S., J. Galeano, J. M. Pastor & M. Méndez, 2014. Robustness of alpine pollination networks: effects of network structure and consequences for endemic plants. Arctic, Antarctic, and Alpine Research 46(3): 568–580.
Strona, G., 2015. Past, present and future of host–parasite co-extinctions. International Journal for Parasitology: Parasites and Wildlife 4(3): 431–441.
Strona, G. & K. D. Lafferty, 2016. Environmental change makes robust ecological networks fragile. Nature Communications 7(1): 12462.
Strona, G., P. Galli & S. Fattorini, 2013. Fish parasites resolve the paradox of missing coextinctions. Nature Communications 4: 1718.
Tylianakis, J. M., E. Laliberté, A. Nielsen & J. Bascompte, 2010. Conservation of species interaction networks. Biological Conservation 143(10): 2270–2279.
Vanbergen, A. J., B. A. Woodcock, M. S. Heard & D. S. Chapman, 2017. Network size, structure and mutualism dependence affect the propensity for plant-pollinator extinction cascades. Functional Ecology 31(6): 1285–1293.
Vázquez, D. P., R. Poulin, B. R. Krasnov & G. I. Shenbrot, 2005. Species abundance and the distribution of specialization in host–parasite interaction networks. Journal of Animal Ecology 74: 946–955.
Ventim, R., J. Morais, S. Pardal, L. Mendes, J. A. Ramos & J. Pérez-Tris, 2012. Host–parasite associations and host-specificity in haemoparasites of reed bed passerines. Parasitology 139: 310–316.
Vieira, M. C. & M. Almeida-Neto, 2015. A simple stochastic model for complex coextinctions in mutualistic networks: robustness decreases with connectance. Ecology Letters 18(2): 144–152.
Walker, J. G., A. Hurford, J. Cable, A. R. Ellison, S. J. Price & C. E. Cressler, 2017. Host allometry influences the evolution of parasite host-generalism: theory and meta-analysis. Philosophical Transactions of the Royal Society B: Biological Sciences 372: 20160089.
Windsor, D. A., 1998. Controversies in parasitology. Most of the species on Earth are parasites. International Journal for Parasitology 28(12): 1939–1941.
Zar, J. R., 2010. Biostatistical Analysis. Pearson Prentice-Hall, Upper Saddle River: 944 pp.
Acknowledgements
We thank the anonymous reviewers for their helpful comments and suggestions on this manuscript. Manuscript funded by Programa de Pós-Graduação em Ecologia de Ambientes Aquáticos Continentais (PEA/UEM), Coordenação de Aperfeiçoamento Pessoal de Nível Superior/Programa de Excelência Acadêmica, Pró-Reitoria de Pesquisa e Pós-Graduação da Universidade Tecnológica Federal do Paraná (PROPPG/UTFPR), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). S.B. was sponsored by Programa Nacional de Pós-doutorado da Coordenação de Aperfeiçoamento Pessoal de Nível Superior (Grant number 88882.315824/2019-00). M.A.N received funding (Grants 310461/2015-4, 408567/2016-3) and R.M.T (Grant number 308197/2018-6) from CNPq.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Eric R. Larson
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bellay, S., de Oliveira, E.F., Almeida-Neto, M. et al. Ectoparasites are more vulnerable to host extinction than co-occurring endoparasites: evidence from metazoan parasites of freshwater and marine fishes. Hydrobiologia 847, 2873–2882 (2020). https://doi.org/10.1007/s10750-020-04279-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-020-04279-x