Abstract
Exfoliated graphite (EG), manganese dioxide (MnO2), and EG/MnO2-based electrodes were used in this work for the degradation of organic pollutants in wastewater under visible light irradiation. Methylene blue and Congo red dyes were used for the degradation. The synthesis of the nanocomposite electrodes was carried out through the co-precipitation technique. The electrodes were engaged for degradation of the dyes under electrochemical oxidation, photolysis, and photoelectrochemical methods. The characterization techniques utilized encompass transmission electron microscopy (TEM), ultraviolet-visible (UV) analysis, scanning electron microscopy (SEM), energy dispersive X-ray (EDX) spectroscopy, Fourier transformed infrared (FTIR) spectroscopy, thermogravimetric analysis (TGA), and X-ray diffraction (XRD). The EG/MnO2 photoanode was applied in the photoelectrochemical degradation of 0.1 × 10–4 M methylene blue and Congo red dyes in 0.1 M Na2SO4 under visible light irradiation. The XRD analysis revealed that the MnO2 exist as α-MnO2. SEM morphologies showed a satisfying dispersion of MnO2 on EG. The EG/MnO2 composite absorbed a noticeable amount of light in the visible light region compared with the pure EG and MnO2. The photoelectrochemical degradation process resulted in enhanced degradation efficiency of Congo red (97.6%) and methylene blue (98.7%) within 60 min and was observed to be higher than those of photolysis and electrochemical oxidation processes.

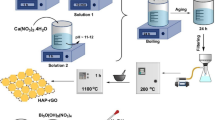
Graphical Abstract








Similar content being viewed by others
References
P. Nigam, I.M. Banat, D. Singh, R. Marchant, Biochem. 31(5), 435–442 (1996)
S.H.S. Chan, W.T. Yeong, J.C. Juan, C.Y. Teh, J. Chem. Technol. Biotechnol. 86(9), 1130–1158 (2011)
G.R.P. Malpass, D.W. Miwa, A.C.P. Miwa, S.A.S. Machado, A.J. Motheo, Environ. Sci. Technol. 41(20), 7120–7125 (2007)
A.S. Stasinakis, J. Global NEST 10(3), 376–385 (2008)
X. Zhao, Y. Zhu, Environ. Sci. Technol. 40(10), 3367–3372 (2006)
R.M. Asmussen, M. Tian, A. Chen, Environ. Sci. Technol. 43(13), 5100–5105 (2009)
S. Malato, P. Fernández-Ibáñez, M.I. Maldonado, J. Blanco, W. Gernjak, Catal. Today 147(1), 1–59 (2009)
O.M. Ama, Int. J. Nano. Med. Eng. 2(8), 145–151 (2017)
G. Hurwitz, P. Pornwongthong, S. Mahendra, E.M. Hoek, J. Chem. Eng. 240, 235–243 (2014)
W. Anku, O.O. Samuel, K.S. Sudheesh, P. Poomani, Acta Chim. Slov. 63(2), 380–391 (2016)
M. Bagheri, R. N. Najme, B.Elham, (2019)
E. Pargoletti, G. Cappelletti, A. Minguzzi, S. Rondinini, M. Leoni, M. Marelli, A. Vertova, High-performance of bare and Ti-doped α-MnO2 nanoparticles in catalyzing the oxygen reduction reaction. J. Power Sources 325, 116–128 (2016)
S. Orsini, E. Pargoletti, A. Vertova, A. Minguzzi, C. Locatelli, S. Rondinini, G. Cappelletti, J. Electron. Chem. 808, 439–445 (2018)
A.B. Djurisič, Y.H. Leung, A.M.C. Ng, Strategies for improving the efficiency of semiconductor metal oxide photocatalysis. Mater. Horiz. 1(4), 400–410 (2014)
M. Ou, J. Huang, X. Yang, K. Quan, Y. Yang, N. Xie, K. Wang, Chem. Sci. 8, 668–673 (2017)
J.T. Liu, X. Ge, X.X. Ye, G.Z. Wang, H.M. Zhang, H.J. Zhou, Y.X. Zhang, H.J. Zhao, J. Mater. Chem. A 4, 1970–1979 (2016)
R. Salunkhe, A.J.H.K. Rahul, Y. Heejoon, Yamauchi, Nanotechnol 26(20), 204004 (2015)
T.L.B. Ferreira, L.M.P. Garcia, G.H.M. Gurgel, R.M. Nascimento, M.J. Godinho, M.H.M. Rodrigues, M.R.D. Bomio, F.V. Motta, J. Mater. Sci. Mater. Electron. 29(14), 12278–12287 (2018)
Y. Chan, S. Leng, Y. Pung, N.S. Hussain, S. Sreekantan, F.Y. Yeoh, Trans. Tech. Pub, 167–174 (2013)
R. Muniba, A. Rehman, S. Rahmat, H.N. Bhatti, M. Iqbal, W.S. Khan, S.Z. Bajwa, R. Rahmat, A. Nazir, J. Mater. Res. Technol. 8(6), 5149–5159 (2019)
M.G. Peleyeju, E.H. Umukoro, L. Tshwenya, R. Moutloali, O. Babalola, O.A. Arotiba, Photoelectrocatalytic water treatment systems: degradation, kinetics and intermediate products studies of sulfamethoxazole on a TiO2–exfoliated graphite electrode. RSC Adv.. 7(64), 40571–40580 (2017)
O.M. Ama, N. Kumar, F.V. Adams, S.S. Ray, Electron, 1–9 (2018)
N. Basahel, T. Sulaiman, T. Ali, Nanoscale Res. Lett. 10(1), 73 (2015)
J. Cao, B. Xu, H. Lin, B. Luo, S. Chen, Dalton Trans. 41(37), 11482–11490 (2012)
J.J. Liu, X.L. Fu, S.F. Chen, Y.F. Zhu, Appl. Phys. Lett. 99(19), 191903 (2011)
W. Gong, X. Meng, X. Ji, P. Tang, Catal 7(1), 19 (2017)
S. Li, X. Lu, Y. Xue, J. Lei, T. Zheng, C. Wang, PLoS One 7(8), 43328 (2012)
X. Liu, L. Pan, T. Lv, Z. Sun, C.Q. Sun, J. Colloid Interface Sci. 408, 145–150 (2013)
B.K. Koo, D.Y. Lee, H.J. Kim, W.J. Lee, J.S. Song, H.J. Kim, J. Electron. 17(1), 79–82 (2006)
H. Wang, Z. Lu, D. Qian, Y. Li, W. Zhang, Nanotechnol 18(11), 115616 (2007)
A. Aleboyeh, M.E. Olya, H. Aleboyeh, Chem. Eng. J. 137(3), 518–524 (2008)
Q. Zhang, C. Li, T. Li. Int. J. Photogr. (2012)
H. Liu, S. Cheng, M. Wu, H. Wu, J. Zhang, L.W.C. Cao, J. Phys. Chem. A 104(30), 7016–7020 (2000)
Acknowledgments
The authors which to acknowledged Kamdem Hugues for assistance of one material used for the experiment also Csir, (PDRF), NRF University of Johannesburg, Vaal University of South Africa.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ama, O.M., Khoele, K., Anku, W.W. et al. Synthesis and Application of MnO2/Exfoliated Graphite Electrodes for Enhanced Photoelectrochemical Degradation of Methylene Blue and Congo Red Dyes in Water. Electrocatalysis 11, 413–421 (2020). https://doi.org/10.1007/s12678-020-00601-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-020-00601-2