Abstract
Purpose
The PI3K/AKT/mTOR pathway is one of the most highly activated cellular signaling pathways in advanced ovarian cancer. Although several PI3K/AKT/mTOR inhibitors have been developed to treat various types of cancer, the antitumor efficacy of many of these compounds against ovarian cancer has remained unclear.
Methods
Here, we tested and compared a panel of 16 PI3K/AKT/mTOR inhibitors (XL765, Miltefosine, Rapamycin, CCI-779, RAD001, FK506, XL147, GSK2110183, IPI-145, GSK2141795, BYL719, GSK458, CAL-101, XL765 analogue SAR245409, Triciribine, and GDC0941) that have entered clinical trials for antitumor activity against ovarian cancer, as well as the front line drug, paclitaxel. Antitumor efficacy was measured in both ovarian cancer cell lines and patient-derived ovarian primary tumor cell lines in vitro and in vivo.
Results
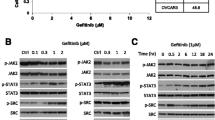
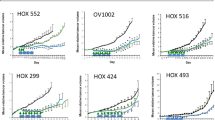
We identified the PI3K/mTOR dual inhibitor GSK458 as a potent inhibitor of proliferation in all cell lines tested at half maximal inhibitory concentrations (IC50) of approximately 0.01-1 µM, a range tens to hundreds fold lower than that of the other PI3K/AKT/mTOR inhibitors tested. Additionally, GSK458 showed the highest inhibitory efficacy against ovarian cancer cell migration. GSK458 also inhibited tumor growth and metastasis in nude mice intraperitoneally engrafted with SKOV3 cells or a patient-derived tumor cell xenograft (PDCX). Importantly, the inhibitory efficiency of GSK458 on cell proliferation and migration both in vitro and in vivo was comparable to that of paclitaxel. Mechanistically, the anti-tumor activity of GSK458 was found to be associated with inactivation of AKT and mTOR, and induction of cell cycle arrest at the G0/G1 phase.
Conclusions
Based on our results, we conclude that GSK458 may serve as an attractive candidate to treat ovarian cancer.





Similar content being viewed by others
References
F. Bray, J. Ferlay, I. Soerjomataram, R.L. Siegel, L.A. Torre, A. Jemal, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018)
R.L. Siegel, K.D. Miller, A. Jemal, Cancer statistics, 2018. CA Cancer J. Clin. 68, 7–30 (2018)
L.A. Torre, F. Bray, R.L. Siegel, J. Ferlay, J. Lortet-Tieulent, A. Jemal, Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108 (2015)
X. Li, M. Tang, Q. Zhu, X. Wang, Y. Lin, X. Wang, The exosomal integrin alpha5beta1/AEP complex derived from epithelial ovarian cancer cells promotes peritoneal metastasis through regulating mesothelial cell proliferation and migration. Cell. Oncol. 43, 263–277 (2020)
S. Vaughan, J.I. Coward, R.C. Bast Jr., A. Berchuck, J.S. Berek, J.D. Brenton, G. Coukos, C.C. Crum, R. Drapkin, D. Etemadmoghadam, M. Friedlander, H. Gabra, S.B. Kaye, C.J. Lord, E. Lengyel, D.A. Levine, I.A. McNeish, U. Menon, G.B. Mills, K.P. Nephew, A.M. Oza, A.K. Sood, E.A. Stronach, H. Walczak, D.D. Bowtell, F.R. Balkwill, Rethinking ovarian cancer: recommendations for improving outcomes. Nat. Rev. Cancer 11, 719–725 (2011)
B.M. Reid, J.B. Permuth, T.A. Sellers, Epidemiology of ovarian cancer: a review. Cancer Biol. Med. 14, 9–32 (2017)
E. Pujade-Lauraine, J.A. Ledermann, F. Selle, V. Gebski, R.T. Penson, A.M. Oza, J. Korach, T. Huzarski, A. Poveda, S. Pignata, M. Friedlander, N. Colombo, P. Harter, K. Fujiwara, I. Ray-Coquard, S. Banerjee, J. Liu, E.S. Lowe, R. Bloomfield, P. Pautier, S.O.E.-O. investigators, Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 18, 1274–1284 (2017)
J. Ledermann, P. Harter, C. Gourley, M. Friedlander, I. Vergote, G. Rustin, C.L. Scott, W. Meier, R. Shapira-Frommer, T. Safra, D. Matei, A. Fielding, S. Spencer, B. Dougherty, M. Orr, D. Hodgson, J.C. Barrett, U. Matulonis, Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 15, 852–861 (2014)
A. Ghahremanloo, A. Soltani, S.M.S. Modaresi, S.I. Hashemy, Recent advances in the clinical development of immune checkpoint blockade therapy. Cell. Oncol. 42, 609–626 (2019)
J.R. Brahmer, S.S. Tykodi, L.Q. Chow, W.J. Hwu, S.L. Topalian, P. Hwu, C.G. Drake, L.H. Camacho, J. Kauh, K. Odunsi, H.C. Pitot, O. Hamid, S. Bhatia, R. Martins, K. Eaton, S. Chen, T.M. Salay, S. Alaparthy, J.F. Grosso, A.J. Korman, S.M. Parker, S. Agrawal, S.M. Goldberg, D.M. Pardoll, A. Gupta, J.M. Wigginton, Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465 (2012)
S.L. Gaillard, A.A. Secord, B. Monk, The role of immune checkpoint inhibition in the treatment of ovarian cancer. Gynecol. Oncol. Res. Pract. 3, 11 (2016)
J. Rodon, R. Dienstmann, V. Serra, J. Tabernero, Development of PI3K inhibitors: lessons learned from early clinical trials. Nat. Rev. Clin. Oncol. 10, 143–153 (2013)
S. Mabuchi, H. Kuroda, R. Takahashi, T. Sasano, The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer. Gynecol. Oncol. 137, 173–179 (2015)
N. Cancer Genome Atlas Research, Integrated genomic analyses of ovarian carcinoma. Nature 474, 609–615 (2011)
M.L. Gasparri, E. Bardhi, I. Ruscito, A. Papadia, A.A. Farooqi, C. Marchetti, G. Bogani, I. Ceccacci, M.D. Mueller, P. Benedetti, Panici, PI3K/AKT/mTOR pathway in ovarian cancer treatment: Are we on the right track? Geburtshilfe Frauenheilkd. 77, 1095–1103 (2017)
H. Li, J. Zeng, K. Shen, PI3K/AKT/mTOR signaling pathway as a therapeutic target for ovarian cancer. Arch. Gynecol. Obstet. 290, 1067–1078 (2014)
D.A. Altomare, H.Q. Wang, K.L. Skele, A. De Rienzo, A.J. Klein-Szanto, A.K. Godwin, J.R. Testa, AKT and mTOR phosphorylation is frequently detected in ovarian cancer and can be targeted to disrupt ovarian tumor cell growth. Oncogene 23, 5853–5857 (2004)
P.L. Bedard, J. Tabernero, F. Janku, Z.A. Wainberg, L. Paz-Ares, J. Vansteenkiste, E. Van Cutsem, J. Perez-Garcia, A. Stathis, C.D. Britten, N. Le, K. Carter, D. Demanse, D. Csonka, M. Peters, A. Zubel, H. Nauwelaerts, C. Sessa, A phase Ib dose-escalation study of the oral pan-PI3K inhibitor buparlisib (BKM120) in combination with the oral MEK1/2 inhibitor trametinib (GSK1120212) in patients with selected advanced solid tumors. Clin. Cancer Res. 21, 730–738 (2015)
T.G. Granda, D. Cebrian, S. Martinez, P.V. Anguita, E.C. Lopez, W. Link, T. Merino, J. Pastor, B.G. Serelde, S. Peregrina, I. Palacios, M.I. Albarran, A. Cebria, M. Lorenzo, P. Alonso, J. Fominaya, A.R. Lopez, J.R. Bischoff, Biological characterization of ETP-46321 a selective and efficacious inhibitor of phosphoinositide-3-kinases. Invest. New Drugs 31, 66–76 (2013)
H. Tanaka, M. Yoshida, H. Tanimura, T. Fujii, K. Sakata, Y. Tachibana, J. Ohwada, H. Ebiike, S. Kuramoto, K. Morita, Y. Yoshimura, T. Yamazaki, N. Ishii, O. Kondoh, Y. Aoki, The selective class I PI3K inhibitor CH5132799 targets human cancers harboring oncogenic PIK3CA mutations. Clin. Cancer Res. 17, 3272–3281 (2011)
O. Treeck, B. Wackwitz, U. Haus, O. Ortmann, Effects of a combined treatment with mTOR inhibitor RAD001 and tamoxifen in vitro on growth and apoptosis of human cancer cells. Gynecol. Oncol. 102, 292–299 (2006)
C.E. Kyriakopoulos, A.M. Braden, J.M. Kolesar, J.C. Eickhoff, H.H. Bailey, J. Heideman, G. Liu, K.B. Wisinski, A phase I study of tivantinib in combination with temsirolimus in patients with advanced solid tumors. Invest. New Drugs 35, 290–297 (2017)
K. Behbakht, M.W. Sill, K.M. Darcy, S.C. Rubin, R.S. Mannel, S. Waggoner, R.J. Schilder, K.Q. Cai, A.K. Godwin, R.K. Alpaugh, Phase II trial of the mTOR inhibitor, temsirolimus and evaluation of circulating tumor cells and tumor biomarkers in persistent and recurrent epithelial ovarian and primary peritoneal malignancies: a Gynecologic Oncology Group study. Gynecol. Oncol. 123, 19–26 (2011)
F. Janku, J.J. Wheler, S.N. Westin, S.L. Moulder, A. Naing, A.M. Tsimberidou, S. Fu, G.S. Falchook, D.S. Hong, I. Garrido-Laguna, R. Luthra, J.J. Lee, K.H. Lu, R. Kurzrock, PI3K/AKT/mTOR inhibitors in patients with breast and gynecologic malignancies harboring PIK3CA mutations. J. Clin. Oncol. 30, 777–782 (2012)
A.C. Wong Te Fong, P. Thavasu, S. Gagrica, K.E. Swales, M.O. Leach, S.C. Cosulich, Y.L. Chung, U. Banerji, Evaluation of the combination of the dual m-TORC1/2 inhibitor vistusertib (AZD2014) and paclitaxel in ovarian cancer models. Oncotarget 8, 113874–113884 (2017)
F. Musa, A. Alard, G. David-West, J.P. Curtin, S.V. Blank, R.J. Schneider, Dual mTORC1/2 inhibition as a novel strategy for the resensitization and treatment of platinum-resistant ovarian cancer. Mol. Cancer Ther. 15, 1557–1567 (2016)
I. Brana, N.A. Pham, L. Kim, S. Sakashita, M. Li, C. Ng, Y. Wang, P. Loparco, R. Sierra, L. Wang, B.A. Clarke, B.G. Neel, L.L. Siu, M.S. Tsao, Novel combinations of PI3K-mTOR inhibitors with dacomitinib or chemotherapy in PTEN-deficient patient-derived tumor xenografts. Oncotarget 8, 84659–84670 (2017)
A. Kudoh, T. Oishi, H. Itamochi, S. Sato, J. Naniwa, S. Sato, M. Shimada, J. Kigawa, T. Harada, Dual inhibition of phosphatidylinositol 3’-kinase and mammalian target of rapamycin using NVP-BEZ235 as a novel therapeutic approach for mucinous adenocarcinoma of the ovary. Int. J. Gynecol. Cancer 24, 444–453 (2014)
T. Kashiyama, K. Oda, Y. Ikeda, Y. Shiose, Y. Hirota, K. Inaba, C. Makii, R. Kurikawa, A. Miyasaka, T. Koso, T. Fukuda, M. Tanikawa, K. Shoji, K. Sone, T. Arimoto, O. Wada-Hiraike, K. Kawana, S. Nakagawa, K. Matsuda, F. McCormick, H. Aburatani, T. Yano, Y. Osuga, T. Fujii, Antitumor activity and induction of TP53-dependent apoptosis toward ovarian clear cell adenocarcinoma by the dual PI3K/mTOR inhibitor DS-7423. PLoS One 9, e87220 (2014)
J.F. Liu, S. Palakurthi, Q. Zeng, S. Zhou, E. Ivanova, W. Huang, I.K. Zervantonakis, L.M. Selfors, Y. Shen, C.C. Pritchard, M. Zheng, V. Adleff, E. Papp, H. Piao, M. Novak, S. Fotheringham, G.M. Wulf, J. English, P.T. Kirschmeier, V.E. Velculescu, C. Paweletz, G.B. Mills, D.M. Livingston, J.S. Brugge, U.A. Matulonis, R. Drapkin, Establishment of patient-derived tumor xenograft models of epithelial ovarian cancer for preclinical evaluation of novel therapeutics. Clin. Cancer Res. 23, 1263–1273 (2017)
M.D. Topp, L. Hartley, M. Cook, V. Heong, E. Boehm, L. McShane, J. Pyman, O. McNally, S. Ananda, M. Harrell, D. Etemadmoghadam, L. Galletta, K. Alsop, G. Mitchell, S.B. Fox, J.B. Kerr, K.J. Hutt, S.H. Kaufmann, E.M. Swisher, D.D. Bowtell, M.J. Wakefield, C.L. Scott, A.O.C. Study, Molecular correlates of platinum response in human high-grade serous ovarian cancer patient-derived xenografts. Mol. Oncol. 8, 656–668 (2014)
S.J. Weroha, M.A. Becker, S. Enderica-Gonzalez, S.C. Harrington, A.L. Oberg, M.J. Maurer, S.E. Perkins, M. AlHilli, K.A. Butler, S. McKinstry, S. Fink, R.B. Jenkins, X. Hou, K.R. Kalli, K.M. Goodman, J.N. Sarkaria, B.Y. Karlan, A. Kumar, S.H. Kaufmann, L.C. Hartmann, P. Haluska, Tumorgrafts as in vivo surrogates for women with ovarian cancer. Clin. Cancer Res. 20, 1288–1297 (2014)
Y.Y. Yangjiong Xiao, D. Gao, W. Jin, P. Jiang, Y. Li et al., Inhibition of CDC25B with WG-391D impedes the tumorigenesis of ovarian cancer. Front. Oncol. 9, 236 (2019)
S.D. Knight, N.D. Adams, J.L. Burgess, A.M. Chaudhari, M.G. Darcy, C.A. Donatelli, J.I. Luengo, K.A. Newlander, C.A. Parrish, L.H. Ridgers, M.A. Sarpong, S.J. Schmidt, G.S. Van Aller, J.D. Carson, M.A. Diamond, P.A. Elkins, C.M. Gardiner, E. Garver, S.A. Gilbert, R.R. Gontarek, J.R. Jackson, K.L. Kershner, L. Luo, K. Raha, C.S. Sherk, C.M. Sung, D. Sutton, P.J. Tummino, R.J. Wegrzyn, K.R. Auger, D. Dhanak, Discovery of GSK2126458, a highly potent inhibitor of PI3K and the mammalian target of rapamycin. ACS Med. Chem. Lett. 1, 39–43 (2010)
P. Munster, R. Aggarwal, D. Hong, J.H. Schellens, R. van der Noll, J. Specht, P.O. Witteveen, T.L. Werner, E.C. Dees, E. Bergsland, N. Agarwal, J.F. Kleha, M. Durante, L. Adams, D.A. Smith, T.A. Lampkin, S.R. Morris, R. Kurzrock, First-inhuman phase I study of GSK2126458, an oral pan-class I phosphatidylinositol-3-kinase inhibitor, in patients with advanced solid tumor malignancies. Clin. Cancer Res. 22, 1932–1939 (2016)
M. Kanehisa, M. Araki, S. Goto, M. Hattori, M. Hirakawa, M. Itoh, T. Katayama, S. Kawashima, S. Okuda, T. Tokimatsu, Y. Yamanishi, KEGG for linking genomes to life and the environment. Nucleic Acids Res. 36, D480–D484 (2008)
S. Domcke, R. Sinha, D.A. Levine, C. Sander, N. Schultz, Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun. 4, 2126 (2013)
C.M. Beaufort, J.C. Helmijr, A.M. Piskorz, M. Hoogstraat, K. Ruigrok-Ritstier, N. Besselink, M. Murtaza, I.W.F. van, A.A. Heine, M. Smid, M.J. Koudijs, J.D. Brenton, E.M. Berns, J. Helleman, Ovarian cancer cell line panel (OCCP): clinical importance of in vitro morphological subtypes. PLoS One 9, e103988 (2014)
H. Yoshida, W. Cheng, J. Hung, D. Montell, E. Geisbrecht, D. Rosen, J. Liu, H. Naora, Lessons from border cell migration in the Drosophila ovary: A role for myosin VI in dissemination of human ovarian cancer. Proc. Natl. Acad. Sci. U. S. A. 101, 8144–8149 (2004)
S. Sale, S. Orsulic, Models of ovarian cancer metastasis: Murine models. Drug Discov. Today Dis. Models 3, 149–154 (2006)
M.A. Eckert, F. Coscia, A. Chryplewicz, J.W. Chang, K.M. Hernandez, S. Pan, S.M. Tienda, D.A. Nahotko, G. Li, I. Blazenovic, R.R. Lastra, M. Curtis, S.D. Yamada, R. Perets, S.M. McGregor, J. Andrade, O. Fiehn, R.E. Moellering, M. Mann, E. Lengyel, Proteomics reveals NNMT as a master metabolic regulator of cancer-associated fibroblasts. Nature 569, 723–728 (2019)
L. Chen, S.M. Park, A.V. Tumanov, A. Hau, K. Sawada, C. Feig, J.R. Turner, Y.X. Fu, I.L. Romero, E. Lengyel, M.E. Peter, CD95 promotes tumour growth. Nature 465, 492–496 (2010)
L. Chen, X. Cheng, W. Tu, Z. Qi, H. Li, F. Liu, Y. Yang, Z. Zhang, Z. Wang, Apatinib inhibits glycolysis by suppressing the VEGFR2/AKT1/SOX5/GLUT4 signaling pathway in ovarian cancer cells. Cell. Oncol. 42, 679–690 (2019)
Z.C. Dobbin, C.N. Landen, The importance of the PI3K/AKT/MTOR pathway in the progression of ovarian cancer. Int. J. Mol. Sci. 14, 8213–8227 (2013)
V.E. Sanchez, C. Nichols, H.N. Kim, E.J. Gang, Y.M. Kim, Targeting PI3K Signaling in Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. (2019). https://doi.org/10.3390/ijms20020412
A. Gul, B. Leyland-Jones, N. Dey, P. De, A combination of the PI3K pathway inhibitor plus cell cycle pathway inhibitor to combat endocrine resistance in hormone receptor-positive breast cancer: a genomic algorithm-based treatment approach. Am. J. Cancer Res. 8, 2359–2376 (2018)
J.J. Lee, K. Loh, Y.S. Yap, PI3K/Akt/mTOR inhibitors in breast cancer. Cancer Biol. Med. 12, 342–354 (2015)
E. Leung, J.E. Kim, G.W. Rewcastle, G.J. Finlay, B.C. Baguley, Comparison of the effects of the PI3K/mTOR inhibitors NVP-BEZ235 and GSK2126458 on tamoxifen-resistant breast cancer cells. Cancer Biol. Ther. 11, 938–946 (2011)
T. Tian, X. Li, J. Zhang, mTOR signaling in cancer and mTOR inhibitors in solid tumor targeting therapy. Int. J. Mol. Sci. (2019). https://doi.org/10.3390/ijms20030755
J.E. Grilley-Olson, P.L. Bedard, A. Fasolo, M. Cornfeld, L. Cartee, A.R. Razak, L.A. Stayner, Y. Wu, R. Greenwood, R. Singh, C.B. Lee, J. Bendell, H.A. Burris, G. Del Conte, C. Sessa, J.R. Infante, A phase Ib dose-escalation study of the MEK inhibitor trametinib in combination with the PI3K/mTOR inhibitor GSK2126458 in patients with advanced solid tumors. Invest. New Drugs 34, 740–749 (2016)
S. Vaidhyanathan, B. Wilken-Resman, D.J. Ma, K.E. Parrish, R.K. Mittapalli, B.L. Carlson, J.N. Sarkaria, W.F. Elmquist, Factors influencing the central nervous system distribution of a novel phosphoinositide 3-kinase/mammalian target of rapamycin inhibitor GSK2126458: Implications for overcoming resistance with combination therapy for melanoma brain metastases. J. Pharmacol. Exp. Ther. 356, 251–259 (2016)
V.A. Kuznetsov, Z. Tang, A.V. Ivshina, Identification of common oncogenic and early developmental pathways in the ovarian carcinomas controlling by distinct prognostically significant microRNA subsets. BMC Genom. 18, 692 (2017)
P. Kanlikilicer, M.H. Rashed, R. Bayraktar, R. Mitra, C. Ivan, B. Aslan, X. Zhang, J. Filant, A.M. Silva, C. Rodriguez-Aguayo, E. Bayraktar, M. Pichler, B. Ozpolat, G.A. Calin, A.K. Sood, G. Lopez-Berestein, Ubiquitous release of exosomal tumor suppressor miR-6126 from ovarian cancer cells. Cancer Res. 76, 7194–7207 (2016)
M.L. Gasparri, Z.M. Besharat, A.A. Farooqi, S. Khalid, K. Taghavi, R.A. Besharat, C. Sabato, A. Papadia, P.B. Panici, M.D. Mueller, E. Ferretti, MiRNAs and their interplay with PI3K/AKT/mTOR pathway in ovarian cancer cells: a potential role in platinum resistance. J. Cancer Res. Clin. Oncol. 144, 2313–2318 (2018)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 81702563 to Y.J.X., and 81472445 and 81672587 to R.Z.), the Postdoctoral Science Foundation of China (Grant No. 2019M652850 to Y.J.X.) and the Scientific and Technological Innovation Act Program of the Shanghai Science and Technology Commission (Grant No. 4411973100 to R.Z.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Written informed consent was obtained from all individual participants according to the Declaration of Helsinki. This study was carried out in accordance with the recommendations of, and approval by the Ethical Committee of Fengxian District Central Hospital. All animal experimental procedures described in this study were performed according to the Guidelines for the care and use of animals established by the Laboratory Animal Ethical Board and approved by the Animal Care and Use committee of the East China Normal University School of Life Sciences.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Xiao, Y., Yu, Y., Jiang, P. et al. The PI3K/mTOR dual inhibitor GSK458 potently impedes ovarian cancer tumorigenesis and metastasis. Cell Oncol. 43, 669–680 (2020). https://doi.org/10.1007/s13402-020-00514-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-020-00514-8