Formation of Nanoclusters in Gold Nucleation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments and Experiment
2.3. Data Analysis
3. Results and Discussion
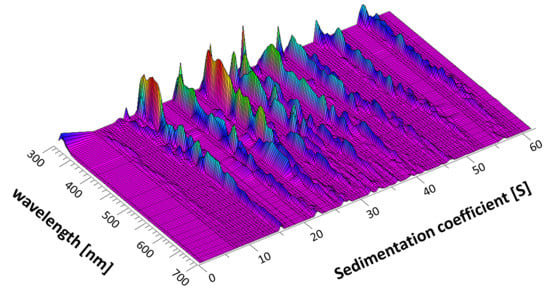
3.1. Analysis Using SEDFIT
3.2. Analysis Using ULTRASCAN
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goswami, N.; Yao, Q.; Chen, T.; Xie, J. Mechanistic exploration and controlled synthesis of precise thiolate-gold nanoclusters. Coord. Chem. Rev. 2016, 329, 1–15. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, W. Sub-nanometre sized metal clusters: From synthetic challenges to the unique property discoveries. Chem. Soc. Rev. 2012, 41, 3594–3623. [Google Scholar] [CrossRef]
- Lee, J.; Yang, J.; Kwon, S.G.; Hyeon, T. Nonclassical nucleation and growth of inorganic nanoparticles. Nat. Rev. Mater. 2016, 1, 16034. [Google Scholar] [CrossRef]
- Shields, S.P.; Richards, V.N.; Buhro, W.E. Nucleation control of size and dispersity in aggregative nanoparticle growth. A study of the coarsening kinetics of thiolate-capped gold nanocrystals. Chem. Mater. 2010, 22, 3212–3225. [Google Scholar] [CrossRef]
- Li, X.-B.; Wang, H.-Y.; Yang, X.-D.; Zhu, Z.-H.; Tang, Y.-J. Size dependence of the structures and energetic and electronic properties of gold clusters. J. Chem. Phys. 2007, 126, 084505. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Zhu, Y.; Jin, R. Atomically precise gold nanocrystal molecules with surface plasmon resonance. Proc. Natl. Acad. Sci. USA 2012, 109, 696–700. [Google Scholar] [CrossRef] [Green Version]
- Barnard, A.S. Modelling of nanoparticles: Approaches to morphology and evolution. Rep. Prog. Phys. 2010, 73, 086502. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Pedersen, A.; Gao, Y.; Khetrapal, N.; Jónsson, H.; Zeng, X.C. Magic-number gold nanoclusters with diameters from 1 to 3.5 nm: Relative stability and catalytic activity for co oxidation. Nano Lett. 2015, 15, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid–liquid system. J. Chem. Soc. Chem. Commun. 1994, 7, 801–802. [Google Scholar] [CrossRef]
- Sakthivel, N.A.; Dass, A. Aromatic thiolate-protected series of gold nanomolecules and a contrary structural trend in size evolution. Acc. Chem. Res. 2018, 51, 1774–1783. [Google Scholar] [CrossRef]
- Olivares, A.; Laskin, J.; Johnson, G.E. Investigating the synthesis of ligated metal clusters in solution using a flow reactor and electrospray ionization mass spectrometry. J. Phys. Chem. A 2014, 118, 8464–8470. [Google Scholar] [CrossRef] [PubMed]
- Ligare, M.R.; Johnson, G.E.; Laskin, J. Observing the real time formation of phosphine-ligated gold clusters by electrospray ionization mass spectrometry. PCCP 2017, 19, 17187–17198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Keller, D.; Rossell, M.D.; Erni, R. Formation of au nanoparticles in liquid cell transmission electron microscopy: From a systematic study to engineered nanostructures. Chem. Mater. 2017, 29, 10518–10525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polte, J.; Erler, R.; Thünemann, A.F.; Sokolov, S.; Ahner, T.T.; Rademann, K.; Emmerling, F.; Kraehnert, R. Nucleation and growth of gold nanoparticles studied via in situ small angle x-ray scattering at millisecond time resolution. ACS Nano 2010, 4, 1076–1082. [Google Scholar] [CrossRef]
- Polte, J.; Ahner, T.T.; Delissen, F.; Sokolov, S.; Emmerling, F.; Thünemann, A.F.; Kraehnert, R. Mechanism of gold nanoparticle formation in the classical citrate synthesis method derived from coupled in situ xanes and saxs evaluation. J. Am. Chem. Soc. 2010, 132, 1296–1301. [Google Scholar] [CrossRef]
- Polte, J.; Herder, M.; Erler, R.; Rolf, S.; Fischer, A.; Würth, C.; Thünemann, A.F.; Kraehnert, R.; Emmerling, F. Mechanistic insights into seeded growth processes of gold nanoparticles. Nanoscale 2010, 2, 2463–2469. [Google Scholar] [CrossRef]
- Polte, J. Fundamental growth principles of colloidal metal nanoparticles—A new perspective. CrystEngComm 2015, 17, 6809–6830. [Google Scholar] [CrossRef] [Green Version]
- Mächtle, W.; Börger, L. Analytical Ultracentrifugation of Polymers and Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Börger, L.; Cölfen, H. Investigation of the efficiencies of stabilizers for nanoparticles by synthetic boundary crystallization ultracentrifugation. Prog. Colloid Polym. Sci. 1999, 113, 23–28. [Google Scholar]
- Börger, L.; Cölfen, H.; Antonietti, M. Synthetic boundary crystallization ultracentrifugation: A new method for the observation of nucleation and growth of inorganic colloids and the determination of stabilizer efficiencies. Colloids Surf. A 2000, 163, 29–38. [Google Scholar] [CrossRef]
- Völkle, C.M.; Gebauer, D.; Cölfen, H. Fd nucleation: High-resolution insights into the early stages of silver nucleation and growth. Faraday Discuss. 2015, 179, 59–77. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.M.; Cölfen, H. High-resolution analysis of small silver clusters by analytical ultracentrifugation. J. Phys. Chem. Lett. 2019, 10, 6558–6564. [Google Scholar] [CrossRef] [PubMed]
- Wawra, S.E.; Pflug, L.; Thajudeen, T.; Kryschi, C.; Stingl, M.; Peukert, W. Determination of the two-dimensional distributions of gold nanorods by multiwavelength analytical ultracentrifugation. Nat. Commun. 2018, 9, 4898. [Google Scholar] [CrossRef] [PubMed]
- Cölfen, H.; Pauck, T. Determination of particle size distributions with angström resolution. Colloid. Polym. Sci. 1997, 275, 175–180. [Google Scholar] [CrossRef]
- Cölfen, H.; Schnablegger, H.; Fischer, A.; Jentoft, F.C.; Weinberg, G.; Schlögl, R. Particle growth kinetics in zirconium sulfate aqueous solutions followed by dynamic light scattering and analytical ultracentrifugation: Implications for thin film deposition. Langmuir 2002, 18, 3500–3509. [Google Scholar] [CrossRef]
- Karabudak, E.; Brookes, E.; Lesnyak, V.; Gaponik, N.; Eychmüller, A.; Walter, J.; Segets, D.; Peukert, W.; Wohlleben, W.; Demeler, B.; et al. Simultaneous identification of spectral properties and sizes of multiple particles in solution with subnanometer resolution. Angew. Chem. Int. Ed. 2016, 55, 11770–11774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, J.; Löhr, K.; Karabudak, E.; Reis, W.; Mikhael, J.; Peukert, W.; Wohlleben, W.; Cölfen, H. Multidimensional analysis of nanoparticles with highly disperse properties using multiwavelength analytical ultracentrifugation. ACS Nano 2014, 8, 8871–8886. [Google Scholar] [CrossRef]
- Bhattacharyya, S.K.; Maciejewska, P.; Börger, L.; Stadler, M.; Gülsün, A.M.; Cicek, H.B.; Cölfen, H. Development of fast fiber based uv-vis multiwavelength detector for an ultracentrifuge. Prog. Colloid Polym. Sci. 2006, 131, 9–22. [Google Scholar]
- Pearson, J.; Walter, J.; Peukert, W.; Cölfen, H. Advanced multiwavelength detection in analytical ultracentrifugation. Anal. Chem. 2018, 90, 1280–1291. [Google Scholar] [CrossRef]
- Pearson, J.; Hofstetter, M.; Dekorsy, T.; Totzeck, M.; Cölfen, H. Design concepts in absorbance optical systems for analytical ultracentrifugation. Analyst 2018, 143, 4040–4050. [Google Scholar] [CrossRef] [Green Version]
- Demeler, B. Ultrascan—A comprehensive data analysis software package for analytical ultracentrifugation experiments. In Analytical Ultracentrifugation: Techniques and Methods; Scott, D.J., Harding, S.E., Rowe, A.J., Eds.; The Royal Society of Chemistry: London, UK, 2005; pp. 210–230. [Google Scholar]
- Schuck, P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys. J. 2000, 78, 1606–1619. [Google Scholar] [CrossRef] [Green Version]
- Stafford, W.F.; Sherwood, P.J. Analysis of heterologous interacting systems by sedimentation velocity: Curve fitting algorithms for estimation of sedimentation coefficients, equilibrium and kinetic constants. Biophys. Chem. 2004, 108, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.J.; Wright, R.T.; Hayes, D.; Sherwood, P.J.; Stafford, W.F. Auc measurements of diffusion coefficients of monoclonal antibodies in the presence of human serum proteins. Biophys. J. 2018, 114, 62a. [Google Scholar] [CrossRef] [Green Version]
- Lebowitz, J.; Teale, M.; Schuck, P.W. Analytical band centrifugation of proteins and protein complexes. Biochem. Soc. Trans. 1998, 26, 745–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorbet, G.; Devlin, T.; Uribe, B.I.H.; Demeler, A.K.; Lindsey, Z.L.; Ganji, S.; Demeler, B. A parametrically constrained optimization method for fitting sedimentation velocity experiments. Biophys. J. 2014, 106, 1741–1750. [Google Scholar] [CrossRef] [Green Version]
- Carney, R.P.; Kim, J.Y.; Qian, H.; Jin, R.; Mehenni, H.; Stellacci, F.; Bakr, O.M. Determination of nanoparticle size distribution together with density or molecular weight by 2d analytical ultracentrifugation. Nat. Commun. 2011, 2, 335. [Google Scholar] [CrossRef] [Green Version]
- Mächtle, W. Schnelle dynamische h2o/percoll-dichtegradienten für mikropartikel in der analytischen ultrazentrifuge. Colloid. Polym. Sci. 1984, 262, 270–282. [Google Scholar] [CrossRef]
- Schneider, C.M.; Cölfen, H. Analytical band centrifugation revisited. Eur. Biophys. J. 2018, 47, 799–807. [Google Scholar] [CrossRef] [Green Version]
- Rockenberger, J.; Tröger, L.; Rogach, A.L.; Tischer, M.; Grundmann, M.; Eychmüller, A.; Weller, H. The contribution of particle core and surface to strain, disorder and vibrations in thiolcapped cdte nanocrystals. J. Chem. Phys. 1998, 108, 7807–7815. [Google Scholar] [CrossRef]
- Vossmeyer, T.; Reck, G.; Schulz, B.; Katsikas, L.; Weller, H. Double-layer superlattice structure built up of cd32s14 (sch2ch (oh) ch3) 36. Cntdot. 4h2o clusters. J. Am. Chem. Soc. 1995, 117, 12881–12882. [Google Scholar] [CrossRef]
- Mori, T.; Hegmann, T. Determining the composition of gold nanoparticles: A compilation of shapes, sizes, and calculations using geometric considerations. J. Nanopart. Res. 2016, 18, 295. [Google Scholar] [CrossRef] [Green Version]
- Schmid, G. The relevance of shape and size of au55 clusters. Chem. Soc. Rev. 2008, 37, 1909–1930. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.M.; Haffke, D.; Cölfen, H. Band sedimentation experiment in analytical ultracentrifugation revisited. Anal. Chem. 2018, 90, 10659–10663. [Google Scholar] [CrossRef] [PubMed]




| Exp. | V(Res.) µL (a) | [HAuCl4] mmol/L (b) | [NaBH4] mmol/L (c) | [Stabilizer] mmol/L (d) |
|---|---|---|---|---|
| A | 1 | 20 | 10 | 19.3 |
| B | 2 (2 eq) | 10 (1 eq) | 10 (1 eq) | 19.3 (1 eq) |
| C | 1 (1 eq) | 20 (1 eq) | 5 (0.5 eq) | 19.3 (1 eq) |
| D | 1 (1 eq) | 10 (0.5 eq) | 10 (1 eq) | 19.3 (1 eq) |
| E | 1 (1 eq) | 30 (1.5 eq) | 10 (1 eq) | 19.3 (1 eq) |
| F | 1 (1 eq) | 5 (0.25 eq) | 10 (1 eq) | 19.3 (1 eq) |
| G | 1 (1 eq) | 10 (0.5 eq) | 10 (1 eq) | 28.9 (1.5 eq) |
| Exp. | H2O:D2O | ρ [g/mL] | η [P] |
|---|---|---|---|
| A,B,D,E,F | 51:149 | 1.0776 | 0.0104387 |
| C | 52:348 | 1.0905 | 0.0106841 |
| G | 103:297 | 1.0776 | 0.0104387 |
| ULTRASCAN | SEDFIT | ||||
|---|---|---|---|---|---|
| Sedimentation Coefficient [S] | Sedimentation Coefficient [S] | ||||
| Exp. A-1 | Exp. A-3 | Exp. C | Exp. A-1 | Exp. A-3 | Exp. C |
| 0.2 | |||||
| 1.3 | |||||
| 2.4 | |||||
| 5.6 | |||||
| 9.9 | 8.5 | 8.5 | |||
| 15.9 | 14.7 | 15.9 | 14.5 | 15.5 | 15.0 |
| 24.0 | 26.3 | 25.7 | 24.7 | 24.6 | 24.7 |
| 30.5 | 31.3 | 34.3 | 31.4 | 31.7 | 32.7 |
| 39.2 | 37.3 | ||||
| 45.7 | 44.3 | 44.0 | 39.4 | 42.4 | |
| 54.4 | 49.3 | 50.7 | 49.3 | ||
| 62.7 | 63.3 | 60.2 | |||
| 76.6 | 74.6 | ||||
| 90.5 | 85.8 | ||||
| 106.1 | 101.3 | ||||
| 124.5 | |||||
| Sedimentation Coefficient [S] | Cluster Density [g/mL] | d Au Core [nm] | Au Atoms | Δd [nm] |
|---|---|---|---|---|
| 1.3 ± 0.04 | 4.82 | 0.46 ± 0.01 | 4 a | - |
| 2.3 ± 0.15 | 6.10 | 0.60 ± 0.01 | 7 | 0.12 |
| 5.6 * | 8.20 | 0.88 * | 13 | 0.04 |
| 7.6 ± 0.30 | 9.04 | 1.02 ± 0.02 | 18 | 0.02 |
| 8.9 ± 0.45 | 9.37 | 1.08 ± 0.03 | 29 | 0.02 |
| 15 ± 0.47 | 10.75 | 1.40 ± 0.02 | 55 | 0.04 |
| 24 ± 0.63 | 11.92 | 1.72 ± 0.02 | 115 | 0.00 |
| 27 ± 0.56 | 12.16 | 1.82 ± 0.02 | 135 | 0.00 |
| 32 ± 0.98 | 12.58 | 1.96 ± 0.02 | 178 | 0.02 |
| 36 ± 0.80 | 12.82 | 2.08 ± 0.03 | 192 | 0.00 |
| 41 ± 1.46 | 13.13 | 2.20 ± 0.04 | 165 | 0.02 |
| 45 ± 0.69 | 13.34 | 2.28 ± 0.01 | 237 | 0.01 |
| 50 ± 1.72 | 13.56 | 2.42 ± 0.04 | 327 | 0.03 |
| 62 ± 1.24 | 14.00 | 2.68 ± 0.03 | 429 | 0.01 |
| 76 ± 1.00 | 14.38 | 2.94 ± 0.02 | 599 | 0.02 |
| 81 ± 0.50 | 14.51 | 3.04 ± 0.01 | 459 | 0.01 |
| 88 ± 2.35 | 14.66 | 3.16 ± 0.04 | 579 | 0.01 |
| 106 ± 2.61 | 15.00 | 3.46 ± 0.04 | 658 | 0.03 |
| 125 * | 15.27 | 3.74 * | 923 | 0.00 |
| 132 * | 15.36 | 3.84 * | 911 | 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneider, C.M.; Cölfen, H. Formation of Nanoclusters in Gold Nucleation. Crystals 2020, 10, 382. https://doi.org/10.3390/cryst10050382
Schneider CM, Cölfen H. Formation of Nanoclusters in Gold Nucleation. Crystals. 2020; 10(5):382. https://doi.org/10.3390/cryst10050382
Chicago/Turabian StyleSchneider, Cornelia M., and Helmut Cölfen. 2020. "Formation of Nanoclusters in Gold Nucleation" Crystals 10, no. 5: 382. https://doi.org/10.3390/cryst10050382