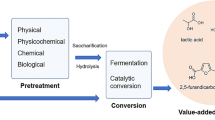
Abstract
In a circular economy, products are made from renewable resources and the waste streams generated during production are either reused, recycled or recovered. The Biocascade methodology considers bio-waste as a resource that can be exploited to produce high-value products such as pharmaceuticals, food ingredients and nutrients; and low-value products such as feed, energy or soil conditioners. The Biocascade principle ensures optimal biomass exploitation by following a hierarchy from high-to-low value, where the waste from one process is the starting material for the next. Biowaste from plant origin is a very suitable resource for applying the Biocascade methodology, both in terms of worldwide production and of variety of components. In this review, the biowaste from sour cherry wine, ornamental kalanchoe plants and red clover feed production, have been examined for processing using a Biocascade approach. Starting from the biowaste characterization, the most relevant components have been identified highlighting their potential uses. The extraction methodology is then discussed in terms of solvent used, operating conditions and yield. Based on the information retrieved from literature, different process flowsheets have been proposed to maximize the use of the biowaste following the Biocascade perspective and targeting zero-waste generation.
Graphic Abstract




Similar content being viewed by others
References
Beeton, I.M., Cookery, M.B., Book: New, and Enlarged Edition., : London. Ward Lock & Co, UK (1899).
Schneider, F. The History Of Food Wastage. in Waste - The Social Context - People, Policies, Persuasion and Payoffs. 2011. Edmonton, Canada.
Batori, V., Lukitawesa, L., Nair, R.: Food waste: Potential source for bioenergy and bio-products. Mycorena AB, Sweden (2017)
Girotto, F., Alibardi, L., Cossu, R.: Food waste generation and industrial uses: A review. Waste Manage. 45, 32–41 (2015)
RedCorn, R., Fatemi, S., Engelberth, A.S.: Comparing End-Use Potential for Industrial Food-Waste Sources. Engineering 4(3), 371–380 (2018)
Kumar, V., Longhurst, P.: Recycling of food waste into chemical building blocks. Current Opinion in Green and Sustainable Chemistry 13, 118–122 (2018)
Bharat Helkar, P., A.K. Sahoo, and N.J. Patil, Review: Food Industry By-Products used as a Functional Food Ingredients. International Journal of Waste Resources, 2016. 6(3): p. 1000248.
Paritosh, K., et al.: Food Waste to Energy: An Overview of Sustainable Approaches for Food Waste Management and Nutrient Recycling. Biomed. Res. Int. 2017, 2370927 (2017)
Baiano, A.: Recovery of Biomolecules from Food Wastes - A Review. Molecules 19(9), 14821–14842 (2014)
European Commission, Directive 2008/98/EC on waste (Waste Framework Directive). 2016.
Md Salim, N.S., A. Singh, and V. Raghavan, Potential Utilization of Fruit and Vegetable Wastes for Food through Drying or Extraction Techniques. Novel Techniques in Nutrition & Food Science, 2017. 1(2).
Galanakis, C.M.: Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 26(2), 68–87 (2012)
Talekar, S., et al.: From waste to wealth: High recovery of nutraceuticals from pomegranate seed waste using a green extraction process. Ind. Crops Prod. 112, 790–802 (2018)
Socaci, S.A., et al., Food Wastes as Valuable Sources of Bioactive Molecules, in Superfood and Functional Food - The Development of Superfoods and Their Roles as Medicine. 2017, IntechOpen.
Ravindran, R., Jaiswal, A.K.: Exploitation of Food Industry Waste for High-Value Products. Trends in Biotechnolgy 34(1), 58–69 (2016)
Galanakis, C.M.: Emerging technologies for the production of nutraceuticals from agricultural by-products: A viewpoint of opportunities and challenges. Food Bioprod. Process. 91(4), 575–579 (2013)
Principato, L., et al.: Adopting the circular economy approach on food loss and waste: The case of Italian pasta production. Resour. Conserv. Recycl. 144, 82–89 (2019)
Zucchella, A., Previtali, P.: Circular business models for sustainable development: A “waste is food” restorative ecosystem. Business Strategy and the Environment 28(2), 274–285 (2018)
Ellen MacArthur Foundation, Toward the Circular Economy: Economic and Business Rationale for Accelerated Transition. 2015, Ellen MacArthur Foundation: Isle of Wight, United Kingdom.
Brar, S.K., S.J. Sarma, and K. Pakshirajan, Platform chemical biorefinery: future green chemistry. 2016: Elsevier.
Venselaar, J.: Sustainable Growth and Chemical Engineering. Chem. Eng. Technol. 26(8), 868–874 (2003)
Venselaar, J., W.F.W.M. van Heugten, and J.J.M. Mulderink, Integrated Plant Conversion and BIOCASCADE optimising use of biomass as renewable resource in sustainable development, in CHISA2000. 2000: Prague.
Kusch-Brandt, S., et al., Valorization of Residues From Beverage Production, in Processing and Sustainability of Beverages. 2019. p. 451–494.
FAOSTAT, Food and Agriculture Organization of the United Nations. 2020; Available from: https://www.fao.org/home/en/.
Nemes, A., et al.: Determination of Flavonoid and Proanthocyanidin Profile of Hungarian Sour Cherry. Molecules 23(12), 3278 (2018)
Muchagato Maurício, E., et al., Evaluation of Industrial Sour Cherry Liquor Wastes as an Ecofriendly Source of Added Value Chemical Compounds and Energy. Waste and Biomass Valorization, 2018.
Švarc-Gajić, J., et al.: Bioactive compounds of sweet and sour cherry stems obtained by subcritical water extraction. J. Chem. Technol. Biotechnol. 93(6), 1627–1635 (2018)
Tumbas Šaponjac, V., et al.: Encapsulation of sour cherry pomace extract by freeze drying: characterization and storage stability. Acta Chim. Slov. 64(2), 283–289 (2017)
Woźniak, Ł., Marszałek, K., Skąpska, S.: Extraction of phenolic compounds from sour cherry pomace with supercritical carbon dioxide: Impact of process parameters on the composition and antioxidant properties of extracts. Sep. Sci. Technol. 51(9), 1472–1479 (2016)
Tumbas Šaponjac, V., et al.: Sour cherry pomace extract encapsulated in whey and soy proteins: Incorporation in cookies. Food Chem 207, 27–33 (2016)
Yilmaz, F.M., M. Karaaslan, and H. Vardin, Optimization of extraction parameters on the isolation of phenolic compounds from sour cherry (Prunus cerasus L.) pomace. Journal of Food Science and Technology, 2015. 52(5): p. 2851–2859.
Adil, İ.H., Yener, M.E., Bayındırlı, A.: Extraction of Total Phenolics of Sour Cherry Pomace by High Pressure Solvent and Subcritical Fluid and Determination of the Antioxidant Activities of the Extracts. Sep. Sci. Technol. 43(5), 1091–1110 (2008)
Rødtjer, A., Skibsted, L.H., Andersen, M.L.: Antioxidative and prooxidative effects of extracts made from cherry liqueur pomace. Food Chem. 99(1), 6–14 (2006)
Petrović, J., et al.: Encapsulated sour cherry pomace extract: Effect on the colour and rheology of cookie dough. Food Sci. Technol. Int. 25(2), 130–140 (2019)
Kopjar, M., et al.: Phenolics Content and Antioxidant Activity of Sour Cherry Extracts with Sugar Addition. Acta Aliment. 46(4), 501–507 (2017)
Issaad, F.Z., et al.: Flavonoids in Selected Mediterranean Fruits: Extraction, Electrochemical Detection and Total Antioxidant Capacity Evaluation. Electroanalysis 29(2), 358–366 (2017)
Ahmad, I., Shamsi, S., Zaman, R.: A review on sour cherry (Prunus cerasus): A high value Unani medicinal fruit. International Journal of Green Pharmacy 11(1), 1–6 (2017)
Homoki, J.R., et al., Anthocyanin composition, antioxidant efficiency, and alpha-amylase inhibitor activity of different Hungarian sour cherry varieties (Prunus cerasus L.). Food Chemistry, 2016. 194: p. 222–229.
Kopjar, M., Orsolic, M., Pilizota, V.: Anthocyanins, Phenols, and Antioxidant Activity of Sour Cherry Puree Extracts and their Stability During Storage. Int. J. Food Prop. 17(6), 1393–1405 (2014)
Piccolella, S., et al., Antioxidant Properties of Sour Cherries (Prunus cerasus L.): Role of Colorless Phytochemicals from the Methanolic Extract of Ripe Fruits. Journal of Agricultural and Food Chemistry, 2008. 55: p. 1928–1935.
Chaovanalikit, A., Wrolstad, R.E.: Total Anthocyanins and Total Phenolics of Fresh and Processed Cherries and Their Antioxidant Properties. Journal of Food Science: Food Chemistry and Toxicology 69(1), 67–72 (2004)
Toydemir, G., et al., Industrial processing effects on phenolic compounds in sour cherry (Prunus cerasus L.) fruit. Food Research International, 2013. 53(1): p. 218–225.
Roda-Serrat, M.C., et al.: Enzyme-Assisted Extraction and Ultrafiltratio of Value-Added Compounds from Sour Cherry Wine Pomace. Chem. Eng. Trans. 74, 811–816 (2019)
Gligor, O., et al.: Enzyme-assisted extractions of polyphenols – A comprehensive review. Trends Food Sci. Technol. 88, 302–315 (2019)
Türkyılmaz, M., Hamzaoğlu, F., Özkan, M.: Effects of sucrose and copigment sources on the major anthocyanins isolated from sour cherries. Food Chem. 281, 242–250 (2019)
Oancea, A.-M., et al.: Functional evaluation of microencapsulated anthocyanins from sour cherries skins extract in whey proteins isolate. LWT - Food Science and Technology 95, 129–134 (2018)
Carvalho, V.V.L., et al.: Separation of anthocyanins extracted from red cabbage by adsorption onto chitosan films. Int. J. Biol. Macromol. 131, 905–911 (2019)
Korlesky, N.M., et al., Extraction and Characterization of Montmorency Sour Cherry (Prunus cerasus L.) Pit Oil. Journal of the American Oil Chemists' Society, 2016. 93(7): p. 995–1005.
Górnaś, P., et al., Composition of bioactive compounds in kernel oils recovered from sour cherry (Prunus cerasus L.) by-products: Impact of the cultivar on potential applications. Industrial Crops and Products, 2016. 82: p. 44–50.
Özcan, M.M., Ünver, A., Arslan, D.: A research on evaluation of some fruit kernels and/or seeds as a raw material of vegetable oil industry. Quality Assurance and Safety of Crops & Foods 7(2), 187–191 (2015)
Yılmaz, C., Gökmen, V.: Compositional characteristics of sour cherry kernel and its oil as influenced by different extraction and roasting conditions. Ind. Crops Prod. 49, 130–135 (2013)
Popa, V.-M., et al.: Characterization of sour cherries (Prunus cerasus) kernel oil cultivars from Banat. Journal of Agroalimentary Processes and Technologies 17(4), 398–401 (2011)
Bak, I., et al.: Isolation and Analysis of Bioactive Constituents of Sour Cherry (Prunus cerasus) Seed Kernel: An Emerging Functional Food. J. Med. Food 13(4), 905–910 (2010)
Matthäus, B., Özcan, M.M.: Fatty Acids and Tocopherol Contents of Some Prunus Spp Kernel Oils. J. Food Lipids 16, 187–199 (2009)
Di Pasquale, M.G.: The Essentials of Essential Fatty Acids. Journal of Dietary Supplements 6(2), 143–161 (2009)
EFSA Panel on Dietetic Products, N.a.A.N., Scientific Opinion on the substantiation of a health claim related to 3 g/day plant sterols/stanols and lowering blood LDL-cholesterol and reduced risk of (coronary) heart disease pursuant to Article 19 of Regulation (EC) No 1924/2006. EFSA Journal, 2012. 10(5): p. 2693–2705.
Salimi, A., et al.: Toxicity Evaluation of Microemulsion (Nano Size) of Sour Cherry Kernel Extract for the Oral Bioavailability. Jundishapur Journal of Natural Pharmaceutical Products 9(1), 16–23 (2014)
Mahmoud, F., et al.: Sour cherry (Prunus cerasus) seed extract increases heme oxygenase-1 expression and decreases proinflammatory signaling in peripheral blood human leukocytes from rheumatoid arthritis patients. Int. Immunopharmacol. 20(1), 188–196 (2014)
Bak, I., et al.: Cardioprotective mechanisms of Prunus cerasus (sour cherry) seed extract against ischemia-reperfusion-induced damage in isolated rat hearts. American Journal of Physiology-Heart and Circulatory Physiology 291(3), H1329–H1336 (2006)
Pap, S., et al.: Evaluation of the adsorption potential of eco-friendly activated carbon prepared from cherry kernels for the removal of Pb2+, Cd2+ and Ni2+ from aqueous wastes. J. Environ. Manage. 184, 297–306 (2016)
Gheju, M., Balcu, I., Jurchescu, P.: Removal of hexavalent chromium from aqueous solutions by use of chemically modified sour cherry stones. Desalination and Water Treatment 57(23), 10776–10789 (2015)
Angin, D.: Utilization of activated carbon produced from fruit juice industry solid waste for the adsorption of Yellow 18 from aqueous solutions. Biores. Technol. 168, 259–266 (2014)
Angin, D.: Production and characterization of activated carbon from sour cherry stones by zinc chloride. Fuel 115, 804–811 (2014)
Švarc-Gajić, J., et al.: Simultaneous dispersive liquid-liquid microextraction derivatisation and gas chromatography mass spectrometry analysis of subcritical water extracts of sweet and sour cherry stems. Anal. Bioanal. Chem. 410(7), 1943–1953 (2018)
Milad, R., El-Ahmadi, S., Singab, A.: Genus Kalanchoe (Crassulaceae): A Review of Its Ethnomedicinal, Botanical, Chemical and Pharmacological Properties. European Journal of Medicinal Plant 4(1), 86–104 (2014)
George, L.O., Radha, H.R., Somasekariah, B.V.: In vitro anti-diabetic activity and GC-MS analysis of bioactive compounds present in the methanol extract of Kalanchoe pinnata. Indian Journal of Chemistry 57B, 1213–1221 (2018)
Madriz-Ordeñana, K., et al.: First report of Kalanchoe leaf and stem spot caused by Corynespora cassiicola in Denmark. Plant Dis. 101(3), 505–505 (2017)
de Araújo, E.R.D., et al.: Local anti-inflammatory activity: Topical formulation containing Kalanchoe brasiliensis and Kalanchoe pinnata leaf aqueous extract. Biomed. Pharmacother. 113, 108721 (2019)
dos Santos Nascimento, L.B., et al.: Optimization of Aqueous Extraction from Kalanchoe pinnata Leaves to Obtain the Highest Content of an Anti-inflammatory Flavonoid using a Response Surface Model. Phytochem. Anal. 29(3), 308–315 (2018)
de Araújo, E.R.D., et al.: Gastroprotective and Antioxidant Activity of Kalanchoe brasiliensis and Kalanchoe pinnata Leaf Juices against Indomethacin and Ethanol-Induced Gastric Lesions in Rats. Int. J. Mol. Sci. 19(5), 1265 (2018)
Bogucka-Kocka, A., et al.: Phenolic acid content, antioxidant and cytotoxic activities of four Kalanchoë species. Saudi Journal of Biological Sciences 25(4), 622–630 (2018)
Fernandes, J.M., et al.: Inhibitory Effects of Hydroethanolic Leaf Extracts of Kalanchoe brasiliensis and Kalanchoe pinnata (Crassulaceae) against Local Effects Induced by Bothrops jararaca Snake Venom. PLoS ONE 11(12), e0168658 (2016)
de Oliveira Costa, A.C., et al.: Quantification of Chemical Marker of Kalanchoe brasiliensis (Crassulaceae) Leaves by HPLC–DAD. J. Liq. Chromatogr. Relat. Technol. 38(7), 795–800 (2015)
El Abdellaoui, S., et al.: Bioactive molecules in Kalanchoe pinnata leaves: extraction, purification, and identification. Anal. Bioanal. Chem. 398(3), 1329–1338 (2010)
Costa, S.S., Corrêa, M.F.P., Casanova, L.M.: A New Triglycosyl Flavonoid Isolated from Leaf Juice of Kalanchoe gastonis-bonnieri (Crassulaceae). Natural Product Communications 10(3), 433–436 (2015)
Nascimento, L.B.D.S., et al.: Ultraviolet-B radiation effects on phenolic profile and flavonoid content of Kalanchoe pinnata. Journal of Photochemistry and Photobiology 148, 73–81 (2015)
Wächter, R., et al.: Leaf press juice from Bryophyllum pinnatum (Lamarck) Oken induces myometrial relaxation. Phytomedicine 19(1), 74–82 (2011)
Indriyanti, N., A. Nuryanti Garmana, and F. Setiawan, Repairing Effects of Aqueous Extract of Kalanchoe pinnata (Lmk) Pers. on Lupus Nephritis Mice. Pharmacognosy Journal, 2018. 10(3): p. 548–552.
Fürer, K., et al.: Two New Flavonol Glycosides and a Metabolite Profile of Bryophyllum pinnatum, a Phytotherapeutic Used in Obstetrics and Gynaecology. Planta Med. 79(16), 1565–1571 (2013)
Sofa, F., et al., Isolation and Identification of Quercetin Derivatives and their α-Glucosidase Inhibitory Activities from Bryophyllum pinnatum. Research Journal of Chemistry and Environment, 2018. 22(II): p. 114–119.
Asiedu-Gyekye, I.J., et al.: Comparative study of two kalanchoe species: Total flavonoid and phenolic contents and antioxidant properties. African Journal of Pure and Applied Chemistry 6(5), 65–73 (2012)
Aisyah, L.S., et al.: Flavonoid Compounds from the Leaves of Kalanchoe prolifera and Their Cytotoxic Activity against P-388 Murine Leukimia Cells. Nat. Prod. Sci. 23(2), 139–145 (2017)
Aisyah, L.S., et al.: Flavonoids from the Fresh Leaves of Kalanchoe tomentosa (Crassulaceae). Open Chemistry Journal 2, 36–39 (2015)
Sharker, S.M., et al., Chemical and biological studies of Kalanchoe pinnata (Lam.) growing in Bangladesh. Asian Pacific Journal of Tropical Biomedicine, 2012. 2(3): p. S1317-S1322.
Rosli, N.H.M., et al.: Determination of Quercetin in Local Kalanchoe Pinnata Extract Using High Performance Liquid Chromatography, in UMT 11th International Annual Symposium of Sustainability Science and Management. Terengganu, Malaysia (2012)
Muzitano, M.F., et al.: Influence of cultivation conditions, season of collection and extraction method on the content of antileishmanial flavonoids from Kalanchoe pinnata. J. Ethnopharmacol. 133(1), 132–137 (2011)
Sohgaura, A.K., Bigoniya, P., Shrivastava, B.: In Vitro Antilithiatic Potential of Kalanchoe pinnata, Emblica officinalis, Bambusa nutans, and Cynodon dactylon. Journal of Pharmacy & BioAllied Sciences 10(2), 83–89 (2018)
Gilhotra, U.K., Mohan, G., Christina, A.J.M.: Antilithiatic activity of poly-herbal formulation tablets by in-vitro method. Journal of Applied Pharmaceutical Science 3(5), 43–48 (2013)
Hamburger, M., et al.: Bryophyllum pinnatum - Reverse Engineering of an Anthroposophic Herbal Medicine. Natural Product Communications 12(8), 1359–1364 (2017)
Kjærgaard, T.: A Plant that Changed the World: The rise and fall of clover 1000–2000. Landscape Research 28(1), 41–49 (2003)
Rasmussen, J., et al.: N2-fixation and residual N effect of four legume species and four companion grass species. Eur. J. Agron. 36(1), 66–74 (2012)
McKenna, P., et al.: The use of red clover ( Trifolium pratense ) in soil fertility-building: A Review. Field Crops Research 221, 38–49 (2018)
Fergus, E. and E. Hollowell, Red clover, in Advances in Agronomy. 1960, Elsevier. p. 365–436.
Weir, W.W., Soil productivity as affected by crop rotation. 1926: US Department of Agriculture.
Van Krimpen, M., et al., Cultivation, processing and nutritional aspects for pigs and poultry of European protein sources as alternatives for imported soybean products. 2013, Wageningen UR Livestock Research.
Boland, M.J., et al.: The future supply of animal-derived protein for human consumption. Trends Food Sci. Technol. 29(1), 62–73 (2013)
Wilkins, R., Jones, R.: Alternative home-grown protein sources for ruminants in the United Kingdom. Anim. Feed Sci. Technol. 85(1–2), 23–32 (2000)
Glencross, R., Festenstein, G., King, H.: Separation and determination of isoflavones in the protein concentrate from red clover leaves. J. Sci. Food Agric. 23(3), 371–376 (1972)
Pirie, N.W., The extended use of fractionation processes. Philosophical Transactions of the Royal Society of London. B, Biological Sciences, 1977. 281(980): p. 139–151.
Mandl, M.G.: Status of green biorefining in Europe. Biofuels, Bioproducts and Biorefining: Innovation for a sustainable economy 4(3), 268–274 (2010)
Damborg, V.K., et al.: Protein value and degradation characteristics of pulp fibre fractions from screw pressed grass, clover, and lucerne. Anim. Feed Sci. Technol. 244, 93–103 (2018)
Hermansen, J.E., et al., Green Biomass-Protein production through bio-refining. 2017: DCA-Nationalt Center for Fødevarer og Jordbrug.
Daems, F., et al., Changes in the isoflavone concentration in red clover (Trifolium pratense L.) during ensiling and storage in laboratory-scale silos. Animal Feed Science and Technology, 2016. 217: p. 36–44.
Solati, Z., et al.: Crude protein yield and theoretical extractable true protein of potential biorefinery feedstocks. Ind. Crops Prod. 115, 214–226 (2018)
Hoekstra, N., et al.: Red clover varieties of Mattenklee type have higher production, protein yield and persistence than Ackerklee types in grass–clover mixtures. Grass Forage Sci. 73(2), 297–308 (2018)
Åman, P.: Chemical composition and in vitro degradability of major chemical constituents in botanical fractions of red clover harvested at different stages of maturity. J. Sci. Food Agric. 36(9), 775–780 (1985)
Grabber, J., Protein fractions in forage legumes containing protein-binding polyphenols: Freeze-drying vs. conservation as hay or silage. Animal feed science and technology, 2009. 151(3–4): p. 324–329.
ESCAA European Seed Certification Agencies Association. Seed production in EU 2018. 2020; Available from: https://www.escaa.org/index/action/page/id/7/title/seed-production-in-eu---2018.
Marshall, T., Clover disease: What do we know and what can we do. Journal of the Department of Agriculture, Western Australia, Series 4, 1973. 14(3): p. 198–206.
Reed, K.: Fertility of herbivores consuming phytoestrogen-containing Medicago and Trifolium species. Agriculture 6(3), 35 (2016)
Mustonen, E., et al., Fertility and growth of nulliparous ewes after feeding red clover silage with high phyto-oestrogen concentrations. animal, 2014. 8(10): p. 1699–1705.
Dornstauder, E., et al.: Estrogenic activity of two standardized red clover extracts (Menoflavon®) intended for large scale use in hormone replacement therapy. The Journal of steroid biochemistry and molecular biology 78(1), 67–75 (2001)
Beck, V., Rohr, U., Jungbauer, A.: Phytoestrogens derived from red clover: an alternative to estrogen replacement therapy? The Journal of steroid biochemistry and molecular biology 94(5), 499–518 (2005)
Atkinson, C., et al.: The effects of phytoestrogen isoflavones on bone density in women: a double-blind, randomized, placebo-controlled trial. The American Journal of clinical nutrition 79(2), 326–333 (2004)
Myers, S.P., Vigar, V.: Effects of a standardised extract of Trifolium pratense (Promensil) at a dosage of 80mg in the treatment of menopausal hot flushes: A systematic review and meta-analysis. Phytomedicine 24, 141–147 (2017)
Coon, J.T., Pittler, M.H., Ernst, E.: Trifolium pratense isoflavones in the treatment of menopausal hot flushes: a systematic review and meta-analysis. Phytomedicine 14(2–3), 153–159 (2007)
Gartoulla, P., Han, M.M.: Red clover extract for alleviating hot flushes in postmenopausal women: a meta-analysis. Maturitas 79(1), 58–64 (2014)
Nestel, P., et al.: A biochanin-enriched isoflavone from red clover lowers LDL cholesterol in men. Eur. J. Clin. Nutr. 58(3), 403 (2004)
Jarred, R.A., et al.: Induction of apoptosis in low to moderate-grade human prostate carcinoma by red clover-derived dietary isoflavones. Cancer Epidemiology and Prevention Biomarkers 11(12), 1689–1696 (2002)
Engelhardt, P.F. and C.R. Riedl, Effects of one-year treatment with isoflavone extract from red clover on prostate, liver function, sexual function, and quality of life in men with elevated PSA levels and negative prostate biopsy findings. Urology, 2008. 71(2): p. 185–90; discussion 190.
Talcott, S.T., Peele, J.E., Brenes, C.H.: Red clover isoflavonoids as anthocyanin color enhancing agents in muscadine wine and juice. Food Res. Int. 38(10), 1205–1212 (2005)
D'Alessandro, T.L., et al.: Metabolism of phytoestrogen conjugates. Methods Enzymol. 400, 316–342 (2005)
Drenin, A.A., Botirov, E.K., Turov, Y.P.: A new isoflavone glycoside from Trifolium pratense L. Russ. J. Bioorg. Chem. 37(7), 862–865 (2011)
Tsao, R., et al.: Isoflavone profiles of red clovers and their distribution in different parts harvested at different growing stages. Journal of agricultural and food chemistry 54(16), 5797–5805 (2006)
Saviranta, N.M., et al., Red clover (Trifolium pratense L.) isoflavones: determination of concentrations by plant stage, flower colour, plant part and cultivar. Journal of the Science of Food and Agriculture, 2008. 88(1): p. 125–132.
Wu, Q., Wang, M., Simon, J.E.: Determination of isoflavones in red clover and related species by high-performance liquid chromatography combined with ultraviolet and mass spectrometric detection. J. Chromatogr. A 1016(2), 195–209 (2003)
Budryn, G., et al., Evaluation of estrogenic activity of red clover (Trifolium pratense L.) sprouts cultivated under different conditions by content of isoflavones, calorimetric study and molecular modelling. Food Chem, 2018. 245: p. 324–336.
Gikas, E., et al.: Determination of isoflavones in the aerial part of red clover by HPLC–diode array detection. J. Liq. Chromatogr. Relat. Technol. 31(8), 1181–1194 (2008)
Sivesind, E., Seguin, P.: Effects of the environment, cultivar, maturity, and preservation method on red clover isoflavone concentration. Journal of Agricultural and Food Chemistry 53(16), 6397–6402 (2005)
Krähmer, A., et al.: Characterization and quantification of secondary metabolite profiles in leaves of red and white clover species by NIR and ATR-IR spectroscopy. Vib. Spectrosc. 68, 96–103 (2013)
He, X.-G., Lin, L.-Z., Lian, L.-Z.: Analysis of flavonoids from red clover by liquid chromatography—electrospray mass spectrometry. J. Chromatogr. A 755(1), 127–132 (1996)
Zgórka, G., Ultrasound‐assisted solid‐phase extraction coupled with photodiode‐array and fluorescence detection for chemotaxonomy of isoflavone phytoestrogens in Trifolium L.(Clover) species. Journal of separation science, 2009. 32(7): p. 965–972.
Klejdus, B., et al.: Supercritical fluid extraction of isoflavones from biological samples with ultra-fast high-performance liquid chromatography/mass spectrometry. J. Sep. Sci. 28(12), 1334–1346 (2005)
Booth, N.L., et al., Seasonal Variation of Red Clover (Trifolium pratense L., Fabaceae) Isoflavones and Estrogenic Activity. Journal of Agricultural and Food Chemistry, 2006. 54(4): p. 1277–1282.
Xu, L., et al.: Production of an isoflavone concentrate from red clover flowers. J. Food Sci. 70(8), s558–s562 (2005)
Xu, L., et al.: Recovery of isoflavones from red clover flowers by a membrane-based process. Innovative Food Science & Emerging Technologies 7(3), 251–256 (2006)
Andres, S., et al.: Determination of the isoflavone composition and estrogenic activity of commercial dietary supplements based on soy or red clover. Food & function 6(6), 2017–2025 (2015)
Luis, B., et al.: Integrated production of ethanol fuel and protein from coastal bermudagrass. Appl. Biochem. Biotechnol. 45(1), 483–497 (1994)
Bals, B., et al.: Extraction of proteins from switchgrass using aqueous ammonia within an integrated biorefinery. Appl. Biochem. Biotechnol. 143(2), 187–198 (2007)
Dale, B.E., et al.: Protein feeds coproduction in biomass conversion to fuels and chemicals. Biofuels, Bioprod. Biorefin. 3(2), 219–230 (2009)
Dotsenko, G., Lange, L.: Enzyme enhanced protein recovery from green biomass pulp. Waste and biomass valorization 8(4), 1257–1264 (2017)
Sari, Y.W., Bruins, M.E., Sanders, J.P.: Enzyme assisted protein extraction from rapeseed, soybean, and microalgae meals. Ind. Crops Prod. 43, 78–83 (2013)
Santamaría-Fernández, M., et al.: Biogas potential of green biomass after protein extraction in an organic biorefinery concept for feed, fuel and fertilizer production. Renewable Energy 129, 769–775 (2018)
El-Naggar, A., et al.: Biochar application to low fertility soils: A review of current status, and future prospects. Geoderma 337, 536–554 (2019)
Acknowledgements
This research work was funded by the European Regional Development Fund as part of the Interreg North Sea Region project 38–2-4–17 BIOCAS, circular BIOmass CAScade to 100%, to whom the authors would like to express their gratitude. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No. 778168.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Madeddu, C., Roda-Serrat, M.C., Christensen, K.V. et al. A Biocascade Approach Towards the Recovery of High-Value Natural Products from Biowaste: State-of-Art and Future Trends. Waste Biomass Valor 12, 1143–1166 (2021). https://doi.org/10.1007/s12649-020-01082-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01082-6