Abstract
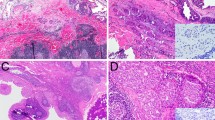
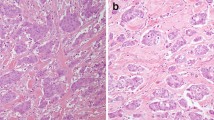
Intraductal carcinoma (IC) is a rare salivary gland tumor with low- to intermediate-grade cytological features. It is further classified into intercalated duct type and apocrine type based on its distinct histologic and immunohistochemical expression. Conventional salivary duct carcinoma (SDC) is an aggressive carcinoma with high-grade features and is usually associated with poor prognosis. In this study, immunohistochemistry and mutation analyses (including HRAS/PIK3CA mutations, RET rearrangement, and human epidermal growth factor receptor 2 [HER2] amplification) of 9 ICs (including 3 pure ICs, 6 ICs with invasive carcinoma) and 24 conventional SDCs were performed and the results were compared. Four intercalated duct-type cases were positive for SOX10 and S100 and negative for AR; five apocrine-type cases showed opposite results. All five apocrine-type cases had cysts with relatively circumscribed tumor borders and morphologically mimicking breast low-grade ductal carcinoma in situ or papillary carcinoma. RET fusion is detected in half of the 4 intercalated duct-type IC but not in the apocrine-type or conventional SDC. HER2 amplification was only observed in conventional SDC. The monoclonal antibody (clone RBT-NRAS) against NRAS Q61R is a sensitive and specific marker used for detecting HRAS Q61R mutation in the salivary gland tumors. The apocrine-type IC had different cytological grades, distinct tumor growth patterns, and no evidence of low- to high-grade transition, suggesting that apocrine-type IC should be distinguished from apocrine SDC with an in situ component.



Similar content being viewed by others
References
Loening T, Leivo I, Simpson RHW, Weinreb I (2017) Intraductal carcinoma. In: El-Naggar AK, Chan JKC, Grandis JR et al (eds) WHO classification of head and neck tumours. IARC Press, Lyon, pp 170–171
Delgado R, Klimstra D, Albores-Saavedra J (1996) Low grade salivary duct carcinoma. A distinctive variant with a low grade histology and a predominant intraductal growth pattern. Cancer 78:958–967
Brandwein-Gensler M, Gnepp DR (2005) Low-grade cribriform cystadenocarcinoma. In: Barnes L, Eveson JW, Reichart P et al (eds) Pathology and genetics head and neck tumors. IARC Press, Lyon, p 233
Kuo YJ, Weinreb I, Perez-Ordonez B (2013) Low-grade salivary duct carcinoma or low-grade intraductal carcinoma? Review of the literature. Head Neck Pathol 7:S59–S67
Weinreb I, Bishop JA, Chiosea SI, Seethala RR, Perez-Ordonez B, Zhang L, Sung YS, Chen CL, Assaad A, Oliai BR, Antonescu CR (2018) Recurrent RET gene rearrangements in intraductal carcinomas of salivary gland. Am J Surg Pathol 42:442–452
Skálová A, Vanecek T, Uro-Coste E, Bishop JA, Weinreb I, Thompson LDR, de Sanctis S, Schiavo-Lena M, Laco J, Badoual C, Santana Conceiçao T, Ptáková N, Baněčkova M, Miesbauerová M, Michal M (2018) Molecular profiling of salivary gland intraductal carcinoma revealed a subset of tumors harboring NCOA4-RET and novel TRIM27-RET fusions: a report of 17 cases. Am J Surg Pathol 42:1445–1455
Skálová A, Ptáková N, Santana T, Agaimy A, Ihrler S, Uro-Coste E, Thompson LDR, Bishop JA, Baněčkova M, Rupp NJ, Morbini P, de Sanctis S, Schiavo-Lena M, Vanecek T, Michal M, Leivo I (2019) NCOA4-RET and TRIM27-RET are characteristic gene fusions in salivary intraductal carcinoma, including invasive and metastatic tumors: is “intraductal” correct? Am J Surg Pathol 43:1303–1313
Hsieh MS, Lee YH, Chang YL (2016) SOX10-positive salivary gland tumors: a growing list, including mammary analogue secretory carcinoma of the salivary gland, sialoblastoma, low-grade salivary duct carcinoma, basal cell adenoma/adenocarcinoma, and a subgroup of mucoepidermoid carcinoma. Hum Pathol 56:134–142
Weinreb I, Tabanda-Lichauco R, Van der Kwast T, Perez-Ordoñez B (2006) Low-grade intraductal carcinoma of salivary gland: report of 3 cases with marked apocrine differentiation. Am J Surg Pathol 30:1014–1021
Kas K, Voz ML, Röijer E, Aström AK, Meyen E, Stenman G et al (1997) Promoter swapping between the genes for a novel zinc finger protein and beta-catenin in pleiomorphic adenomas with t(3;8)(p21;q12) translocations. Nat Genet 15:170–174
Martins C, Fonseca I, Roque L, Pereira T, Ribeiro C, Bullerdiek J, Soares J (2005) PLAG1 gene alterations in salivary gland pleomorphic adenoma and carcinoma ex-pleomorphic adenoma: a combined study using chromosome banding, in situ hybridization and immunocytochemistry. Mod Pathol 18:1048–1055
Antonescu CR, Katabi N, Zhang L, Sung YS, Seethala RR, Jordan RC, Perez-Ordoñez B, Have C, Asa SL, Leong IT, Bradley G, Klieb H, Weinreb I (2011) EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosom Cancer 50:559–570
Persson M, Andrén Y, Mark J, Horlings HM, Persson F, Stenman G (2009) Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci U S A 106:18740–18744
Skálová A, Vanecek T, Sima R, Laco J, Weinreb I, Perez-Ordonez B, Starek I, Geierova M, Simpson RHW, Passador-Santos F, Ryska A, Leivo I, Kinkor Z, Michal M (2010) Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol 34:599–608
Tonon G, Modi S, Wu L, Kubo A, Coxon AB, Komiya T, O’Neil K, Stover K, el-Naggar A, Griffin JD, Kirsch IR, Kaye FJ (2003) t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet 33:208–213
Weinreb I, Zhang L, Tirunagari LM, Sung YS, Chen CL, Perez-Ordonez B et al (2014) Novel PRKD gene rearrangements and variant fusions in cribriform adenocarcinoma of salivary gland origin. Genes Chromosom Cancer 53:845–856
Weinreb I, Piscuoglio S, Martelotto LG, Waggott D, Ng CK, Perez-Ordonez B et al (2014) Hotspot activating PRKD1 somatic mutations in polymorphous low-grade adenocarcinomas of the salivary glands. Nat Genet 46:1166–1169
Chiosea SI, Williams L, Griffith CC, Thompson LD, Weinreb I, Bauman JE et al (2015) Molecular characterization of apocrine salivary duct carcinoma. Am J Surg Pathol 39:744–752
Di Palma S, Simpson RH, Marchiò C, Skálová A, Ungari M, Sandison A et al (2012) Salivary duct carcinomas can be classified into luminal androgen receptor-positive, HER2 and basal-like phenotypes. Histopathology 61:629–643
Khoo TK, Yu B, Smith JA, Clarke AJ, Luk PP, Selinger CI, Mahon KL, Kraitsek S, Palme C, Boyer MJ, Dinger ME, Cowley MJ, O’Toole SA, Clark JR, Gupta R (2017) Somatic mutations in salivary duct carcinoma and potential therapeutic targets. Oncotarget 8:75893–75903
Luk PP, Weston JD, Yu B, Selinger CI, Ekmejian R, Eviston TJ, Lum T, Gao K, Boyer M, O’Toole SA, Clark JR, Gupta R (2016) Salivary duct carcinoma: Clinicopathologic features, morphologic spectrum, and somatic mutations. Head Neck 38:E1838–E1847
Lasota J, Kowalik A, Felisiak-Golabek A, Inaguma S, Wang ZF, Pięciak L, Zięba S, Pęksa R, Kopczynski J, Okoń K, Waloszczyk P, Gozdz S, Biernat W, Miettinen M (2017) SP174, NRAS Q61R mutant-specific antibody, cross-reacts with KRAS Q61R mutant protein in colorectal carcinoma. Arch Pathol Lab Med 141:564–568
Felisiak-Goląbek A, Inaguma S, Kowalik A, Wasąg B, Wang ZF, Zięba S, Pięciak L, Ryś J, Kopczynski J, Sarlomo-Rikala M, Góźdź S, Lasota J, Miettinen M (2018) SP174 antibody lacks specificity for NRAS Q61R and cross-reacts with HRAS and KRAS Q61R mutant proteins in malignant melanoma. Appl Immunohistochem Mol Morphol 26:40–45
Simpson RH (2013) Salivary duct carcinoma: new developments – morphological variants including pure in situ high grade lesions; proposed molecular classification. Head Neck Pathol 7:S48–S58
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, Jenkins RB, Press MF, Spears PA, Vance GH, Viale G, McShane LM, Dowsett M (2018) Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol 36:2105–2122
Tsai TH, Wu SG, Hsieh MS, Yu CJ, Yang JC, Shih JY (2015) Clinical and prognostic implications of RET rearrangements in metastatic lung adenocarcinoma patients with malignant pleural effusion. Lung Cancer 88:208–214
Kusafuka K, Kawasaki T, Maeda M, Yamanegi K, Baba S, Ito Y, Inagaki H, Nakajima T (2017) Salivary duct carcinoma with rhabdoid features: a salivary counterpart of pleomorphic lobular carcinoma of the breast. Histopathology 70:164–173
Williams MD, Roberts DB, Kies MS, Mao L, Weber RS, El-Naggar AK (2010) Genetic and expression analysis of HER-2 and EGFR genes in salivary duct carcinoma: empirical and therapeutic significance. Clin Cancer Res 16:2266–2274
Limaye SA, Posner MR, Krane JF, Fonfria M, Lorch JH, Dillon DA, Shreenivas AV, Tishler RB, Haddad RI (2013) Trastuzumab for the treatment of salivary duct carcinoma. Oncologist 18:294–300
Boon E, Bel M, van Boxtel W, van der Graaf WTA, van Es RJJ, Eerenstein SEJ, Baatenburg de Jong RJ, van den Brekel MWM, van der Velden LA, Witjes MJH, Hoeben A, Willems SM, Bloemena E, Smit LA, Oosting SF, PALGA Group, Jonker MA, Flucke UE, van Herpen CML (2018) A clinicopathological study and prognostic factor analysis of 177 salivary duct carcinoma patients from the Netherlands. Int J Cancer 143:758–766
Gilbert MR, Sharma A, Schmitt NC, Johnson JT, Ferris RL, Duvvuri U, Kim S (2016) A 20-year review of 75 cases of salivary duct carcinoma. JAMA Otolaryngol Head Neck Surg 142:489–495
Omholt K, Karsberg S, Platz A, Kanter L, Ringborg U, Hansson J (2002) Screening of N-ras codon 61 mutations in paired primary and metastatic cutaneous melanomas: mutations occur early and persist throughout tumor progression. Clin Cancer Res 8:3468–3474
Nikiforov YE, Nikiforova MN, Gnepp DR, Fagin JA (1996) Prevalence of mutations of ras and p53 in benign and malignant thyroid tumors from children exposed to radiation after the Chernobyl nuclear accident. Oncogene 13:687–693
Acknowledgments
The authors would like to thank Ms. Syue-Fong Hua for her technical support and Pei-Wen Yang, PhD, for statistical assistance.
Funding
This study was supported by grants from the National Taiwan University Hospital (nos. 106-M3739, 106-003612, 107-N3971, and 107-N4038).
Author information
Authors and Affiliations
Contributions
MSH, YTJ, and YJK designed the research study. MSH and YHL performed the research. MSH and YHL analyzed the data. MSH and YJK wrote the paper.
Corresponding author
Ethics declarations
Clinical data of these cases were obtained from their medical records. This study (201812013RINA) was approved by the Research Ethics Committee of National Taiwan University Hospital.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hsieh, MS., Lee, YH., Jin, YT. et al. Clinicopathological study of intraductal carcinoma of the salivary gland, with emphasis on the apocrine type. Virchows Arch 477, 581–592 (2020). https://doi.org/10.1007/s00428-020-02823-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-020-02823-7