Abstract
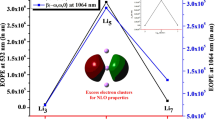
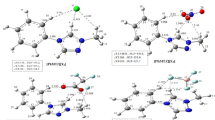
The interesting features in the lithium based electride motived us to explore new species with electride properties. To achieve this goal, the tetracyano-2,6-naphthoquinodimethane (TNAP) species has been used as backbone to investigate systematic addition of lithium atoms to the TNAP backbone (Lin@TNAP (n = 1–4) species) through density functional theory (DFT) simulation. After finding the most stable geometries for each Lin@TNAP (n = 1–4) species by full optimization process, we show their electronic-structural features in this work. In the next step, the properties of electron-density-laplacian (∇2ρ(r)), non-linear-optical (NLO), non-nuclear-attractor (NNA), and electron-localization-function (ELF) have been studied to incorporate the reported Lin@TNAP (n = 1–4) species in two different categories, salt or electride. The obtained outcomes present that the Li1@TNAP and the Li2@TNAP molecules are the lithium-salt. In contrast, the Li3@TNAP and the Li4@TNAP molecules are lithium-based electrides along with the isolated electrons in the molecular structure.





Similar content being viewed by others
References
Zurek E, Edwards PP (2009) Hoffmann R. A molecular perspective on lithium–ammonia solutions. Angew Chem Int Ed. 48:8198–8232
Dye JL (2003) Electrons as anions. Science. 301:607–608
Dye JL (1969) Metal-ammonia solutions, Colloque Weyl II, Ithaca, New York. Butterworths, London, pp 1–17
Dye JL, DeBacker MG, Dorfman LM (1970) Pulse radiolysis studies XVIII Spectrum of the solvated electron in the systems ethylenediamine–water and ammonia–water. J Chem Phys 52:6251–6258
Dye JL (1990) Electrides: ionic salts with electrons as the anions. Science 247:663–668
Kaplan TA, Harrison JF, Dye JL, Rencsok R (1995) Relation of Li(NH3)4 to electrides. Phys Rev Lett 75:978–978
Dye JL (1991) Electrides and alkalides-comparison with metal solutions. J Phys IV 1:259–282
Toda Y, Hirayama H, Kuganathan N, Torrisi A, Sushko PV, Hosono H (2013) Activation and splitting of carbon dioxide on the surface of an inorganic electride material. Nat Commun 4:2378–2385
Kitano M, Inoue Y, Yamazaki Y, Hayashi F, Kanbara S, Matsuishi S, Yokoyama T, Kim S-W, Hara M, Hosono H (2012) Ammonia synthesis using a stable electride as an electron donor and reversible hydrogen store. Nat Chem 4:934–940
Kitano M, Kanbara S, Inoue Y, Kuganathan N, Sushko PV, Yokoyama T, Hara M, Hosono H (2015) Electride support boosts nitrogen dissociation over ruthenium catalyst and shifts the bottleneck in ammonia synthesis. Nat Commum 6:6731–6739
Lu Y, Li J, Tada T, Toda Y, Ueda S, Yokoyama T, Kitano M, Hosono H (2016) Water durable electride Y5Si3: electronic structure and catalytic activity for ammonia synthesis. J Am Chem Soc 138:3970–3973
Dye JL (1993) Anionic electrons in electrides. Nature. 365:10–11
Singh DJ, Krakauer H, Haas C, Pickett WE (1993) Theoretical determination that electrons act as anions in the electride Cs+ (15-crown-5)2·e−. Nature 365:39–42
Dye JL (1997) Electrides: from 1D Heisenberg chains to 2D pseudo-metals. Inorg Chem 36:3816–3826
Becke AD, Edgecombe KE (1990) A simple measure of electron localization in atomic and molecular systems. J Chem Phys 92:5397–5403
Marqués M, Ackland GJ, Lundegaard LF, Stinton G, Nelmes RJ, McMahon MI, Contreras-García J (2009) Potassium under pressure: a pseudobinary ionic compound. Phys Rev Lett 103:115501–1155014
Lee K, Kim SW, Toda Y, Matsuishi S, Hosono H (2013) Dicalcium nitride as a two-dimensional electride with an anionic electron layer. Nature. 494:336–340
Dale SG, Otero-de-la Roza A, Johnson ER (2014) Density-functional description of electrides. Phys Chem Chem Phys 16:14584–14593
Garcia-Borràs M, Solà M, Luis JM, Kirtman B (2012) Electronic and vibrational nonlinear optical properties of five representative electrides. J Chem Theory Comput 8:2688–2697
Chen W, Li Z-R, Wu D, Li Y, Sun C-C, Gu FL (2005) The structure and the large nonlinear optical properties of Li@calix[4]pyrrole. J Am Chem Soc 127:10977–10981
Wang Y-F, Li Z-R, Wu D, Sun C-C, Gu F-L (2010) Excess electron is trapped in a large single molecular cage C60F60. J Comput Chem 31:195–203
Torkpoor I, Heidari Nezhad Janjanpour M, Salehi N, Gharibzadeh F, Edjlali L (2018) Insight into Y@X2B8 (Y = Li, CO2 and Li-CO2, X = Be, B and C) nanostructures: a computational study. Chem Rev Lett 1:2–8
Gharibzadeh F, Gohari S, Nejati K, Hashemzadeh B, Mohammadiyan S (2018) The Be atom doping: an effective way to improve the Li-atom adsorption in boron rich nanoflake of B24. Chem Rev Lett 1:16–22
Postils V, Garcia-Borràs M, Solà M, Luis JM, Matito E (2015) On the existence and characterization of molecular electrides. Chem Commun 51:4865–4868
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) General atomic and molecular electronic structure system. J Comput Chem 14:1347–1363
Adamo C, Barone V (1999) Toward reliable density functional methods without adjustable parameters: the PBE0 model. J Chem Phys 110:6158–6170
Grimme S, Antony J, Ehrlich S, Krieg S (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104–154122
Edwards PP, Anderson PA, Thomas JM (1996) Dissolved alkali metals in zeolites. Acc Chem Res 29:23–29
Champagne B, Perpete EA, Jacquenmin D, van Gisbergen SJA, Baerends EJ, Soubra-Ghaoui C, Robins KA, Kirtman B (2000) Assessment of conventional density functional schemes for computing the dipole moment and (hyper)polarizabilities of push−pull π-conjugated systems. J Phys Chem A 104:4755–4763
Champagne B, Botek E, Nakano M, Nitta T, Yamaguchi K (2005) Basis set and electron correlation effects on the polarizability and second hyperpolarizability of model open-shell ππ-conjugated systems. J Chem Phys 122:114315–114326
Nakano M, Kishi R, Nitta T, Kubo T, Nakasuji K, Kamada K, Ohta K, Champagne B, Botek E, Yamaguchi K (2005) Second hyperpolarizability (γ) of singlet diradical system: dependence of γ on the diradical character. J Phys Chem A 109:885–891
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–590
Bader RFW (1990) In: Halpen J, Green MLH (eds) The international series of monographs of chemistry. Clarendon Press, Oxford
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2489 kb).
Rights and permissions
About this article
Cite this article
Vessally, E., Majedi, S., Hosseinian, A. et al. Cavity-trapped electrons: lithium doped tetracyano-2,6-naphthoquinodimethane (TNAP) systems. J Mol Model 26, 118 (2020). https://doi.org/10.1007/s00894-020-04384-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04384-7