Abstract
Introduction
Conversion in the metabolism of drugs occurs in diabetes mellitus. Considering the importance of metabolic enzymes’ activities on the efficacy and safety of medicines, the changes in liver enzymatic activity of CYP2D1 and its related hepatic clearance, by using Dextromethorphan as probe in the animal model of type I and type II diabetes, before and after treatment, was assessed in this study.
Methods
Male Wistar rats were randomly divided into 6 groups. Seven days after induction of diabetes type I and type II, treatment groups were received insulin and metformin daily for 14 days, respectively. In day 21, rats were subjected to liver perfusion by Krebs-Henseleit buffer containing Dextromethorphan as CYP2D1 probe. Perfusate samples were analyzed by HPLC fluorescence method in order to evaluate any changes in CYP2D1 activity.
Results
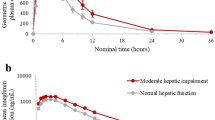
The average metabolic ratio of dextromethorphan and hepatic clearance were changed from 0.012 ± 0.004 and 6.3 ± 0.1 in the control group to 0.006 ± 0.0008 and 5.2 ± 0.2 in the untreated type I diabetic group, and 0.008 ± 0.003 and 5.0 ± 0.6 in the untreated type II diabetic rats. Finally, the mean metabolic ratio and hepatic clearance were changed to 0.008 ± 0.001 and 5.4 ± 0.1, and 0.013 ± 0.003 and 6.1 ± 0.4 in the treated groups with insulin and metformin, respectively.
Conclusion
In type I diabetic rats, corresponding treatment could slightly improve enzyme activity, whereas the hepatic clearance and enzyme activity reached to the normal level in type II group.

.



Similar content being viewed by others
References
Huang D, Refaat M, Mohammedi K, Jayyousi A, Al Suwaidi J, Abi KC. Macrovascular complications in patients with diabetes and Prediabetes. Biomed Res Int. 2017;2017:7839101–9. https://doi.org/10.1155/2017/7839101.
Migdalis I, Czupryniak L, Lalic N, Leslie RD, Papanas N, Valensi P. Diabetic microvascular complications. Int J Endocrinol. 2018;2018:5683287–2. https://doi.org/10.1155/2018/5683287.
Bassil MS, Gougeon R. Muscle protein anabolism in type 2 diabetes. Curr Opin Clin Nutr Metab Care. 2013;16(1):83–8. https://doi.org/10.1097/MCO.0b013e32835a88ee.
Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol. 2017;13(10):572–87. https://doi.org/10.1038/nrendo.2017.80.
Sun X, Haas ME, Miao J, Mehta A, Graham MJ, Crooke RM, et al. Insulin dissociates the effects of liver X receptor on Lipogenesis, endoplasmic reticulum stress, and inflammation. J Biol Chem. 2016;291(3):1115–22. https://doi.org/10.1074/jbc.M115.668269.
Peron EP, Ogbonna KC, Donohoe KL. Antidiabetic medications and polypharmacy. Clin Geriatr Med. 2015;31(1):17–27, vii. https://doi.org/10.1016/j.cger.2014.08.017.
Tang W, Lu AY. Metabolic bioactivation and drug-related adverse effects: current status and future directions from a pharmaceutical research perspective. Drug Metab Rev. 2010;42(2):225–49. https://doi.org/10.3109/03602530903401658.
Kotzamanis K, Angulo A, Ghazal P. Infection homeostasis: implications for therapeutic and immune programming of metabolism in controlling infection. Med Microbiol Immunol. 2015;204(3):395–407. https://doi.org/10.1007/s00430-015-0402-5.
Wu W, Zhao S. Metabolic changes in cancer: beyond the Warburg effect. Acta Biochim Biophys Sin Shanghai. 2013;45(1):18–26. https://doi.org/10.1093/abbs/gms104.
Dogan Y, Akarsu S, Ustundag B, Yilmaz E, Gurgoze MK. Serum IL-1beta, IL-2, and IL-6 in insulin-dependent diabetic children. Mediat Inflamm. 2006;2006(1):59206–6. https://doi.org/10.1155/MI/2006/59206.
Feng S, Yu H, Yu Y, Geng Y, Li D, Yang C, et al. Levels of inflammatory cytokines IL-1beta, IL-6, IL-8, IL-17A, and TNF-alpha in aqueous humour of patients with diabetic retinopathy. J Diabetes Res. 2018;2018:8546423–6. https://doi.org/10.1155/2018/8546423.
Nebert DW, Wikvall K, Miller WL. Human cytochromes P450 in health and disease. Philos Trans R Soc Lond Ser B Biol Sci. 2013;368(1612):20120431. https://doi.org/10.1098/rstb.2012.0431.
Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138(1):103–41. https://doi.org/10.1016/j.pharmthera.2012.12.007.
Neyshaburinezhad N, Rouini M, Entezari H, Lavasani H, Hosseinzadeh Ardakani Y. Evaluation of changes in cytochrome P450 2C19 activity in type 2 diabetic rats before and after treatment, by using isolated perfused liver model. Iran J Basic Med Sci. 2020;23:629–35. doi:https://doi.org/10.22038/ijbms.2020.40836.9642.
Stingl JC, Brockmoller J, Viviani R. Genetic variability of drug-metabolizing enzymes: the dual impact on psychiatric therapy and regulation of brain function. Mol Psychiatry. 2013;18(3):273–87. https://doi.org/10.1038/mp.2012.42.
Martignoni M, Groothuis GM, de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol. 2006;2(6):875–94. https://doi.org/10.1517/17425255.2.6.875.
Tyndale RF, Li Y, Li NY, Messina E, Miksys S, Sellers EM. Characterization of cytochrome P-450 2D1 activity in rat brain: high-affinity kinetics for dextromethorphan. Drug Metab Dispos. 1999;27(8):924–30.
Ghasemi A, Khalifi S, Jedi S. Streptozotocin-nicotinamide-induced rat model of type 2 diabetes (review). Acta Physiol Hung. 2014;101(4):408–20. https://doi.org/10.1556/APhysiol.101.2014.4.2.
Wu J, Yan LJ. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic beta cell glucotoxicity. Diabetes Metab Syndr Obes. 2015;8:181–8. https://doi.org/10.2147/DMSO.S82272.
Jamshidfar S, Ardakani YH, Lavasani H, Rouini M. Inhibition of mirtazapine metabolism by ecstasy (MDMA) in isolated perfused rat liver model. Daru. 2017;25(1):16. https://doi.org/10.1186/s40199-017-0183-z.
Magalhaes P, De Andres F, Falcao A, A LL, Alves G. Can the CEIBA Cocktail Designed for Human Cytochrome P450 Enzymes be Used in the Rat for Drug Interaction Studies? J Pharm Pharm Sci. 2016;19(4):520–9. doi:https://doi.org/10.18433/J3D313.
Wojtczak A, Rychlik-Sych M, Krochmalska-Ulacha E, Skretkowicz J. CYP2D6 phenotyping with dextromethorphan. Pharmacol Rep. 2007;59(6):734–8.
Gaedigk A, Dinh JC, Jeong H, Prasad B, Leeder JS. Ten Years' Experience with the CYP2D6 Activity Score: A Perspective on Future Investigations to Improve Clinical Predictions for Precision Therapeutics. J Pers Med. 2018;8(2). doi:https://doi.org/10.3390/jpm8020015.
Clark M, Kroger CJ, Tisch RM. Type 1 diabetes: a chronic anti-self-inflammatory response. Front Immunol. 2017;8:1898. https://doi.org/10.3389/fimmu.2017.01898.
Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98–107. https://doi.org/10.1038/nri2925.
Lontchi-Yimagou E, Sobngwi E, Matsha TE, Kengne AP. Diabetes mellitus and inflammation. Curr Diab Rep. 2013;13(3):435–44. https://doi.org/10.1007/s11892-013-0375-y.
Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis GA, Vogiatzi G, Papaioannou S et al. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur Cardiol. 2019;14(1):50–9. doi:https://doi.org/10.15420/ecr.2018.33.1.
Kebis A, Kukan M, Grancic P, Jakubovsky J. A novel way of liver preservation improves rat liver viability upon reperfusion. J Zhejiang Univ Sci B. 2007;8(5):289–95. https://doi.org/10.1631/jzus.2007.B0289.
Lin SY, Chen CH, Ho HO, Chen HH, Sheu MT. Simultaneous analysis of dextromethorphan and its three metabolites in human plasma using an improved HPLC method with fluorometric detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;859(1):141–6. https://doi.org/10.1016/j.jchromb.2007.09.017.
Mehvar R. Application of organ clearance to estimation of the in vivo hepatic extraction ratio. Curr Clin Pharmacol. 2016;11(1):47–52.
Schmith VD, Foss JF. Effects of inflammation on pharmacokinetics/pharmacodynamics: increasing recognition of its contribution to variability in response. Clin Pharmacol Ther. 2008;83(6):809–11. https://doi.org/10.1038/clpt.2008.62.
Almazroo OA, Miah MK, Venkataramanan R. Drug metabolism in the liver. Clin Liver Dis. 2017;21(1):1–20. https://doi.org/10.1016/j.cld.2016.08.001.
Galetin A, Houston JB. Intestinal and hepatic metabolic activity of five cytochrome P450 enzymes: impact on prediction of first-pass metabolism. J Pharmacol Exp Ther. 2006;318(3):1220–9. https://doi.org/10.1124/jpet.106.106013.
Gandhi A, Moorthy B, Ghose R. Drug disposition in pathophysiological conditions. Curr Drug Metab. 2012;13(9):1327–44.
Shah RR, Smith RL. Inflammation-induced phenoconversion of polymorphic drug metabolizing enzymes: hypothesis with implications for personalized medicine. Drug Metab Dispos. 2015;43(3):400–10. https://doi.org/10.1124/dmd.114.061093.
Morgan ET. Impact of infectious and inflammatory disease on cytochrome P450-mediated drug metabolism and pharmacokinetics. Clin Pharmacol Ther. 2009;85(4):434–8. https://doi.org/10.1038/clpt.2008.302.
Negro F, Forton D, Craxi A, Sulkowski MS, Feld JJ, Manns MP. Extrahepatic morbidity and mortality of chronic hepatitis C. Gastroenterology. 2015;149(6):1345–60. https://doi.org/10.1053/j.gastro.2015.08.035.
Sakuma T, Honma R, Maguchi S, Tamaki H, Nemoto N. Different expression of hepatic and renal cytochrome P450s between the streptozotocin-induced diabetic mouse and rat. Xenobiotica. 2001;31(4):223–37. https://doi.org/10.1080/00498250110046451.
Gravel S, Chiasson JL, Turgeon J, Grangeon A, Michaud V. Modulation of CYP450 activities in patients with type 2 diabetes. Clin Pharmacol Ther. 2019;106:1280–9. https://doi.org/10.1002/cpt.1496.
Abdel-Razzak Z, Loyer P, Fautrel A, Gautier JC, Corcos L, Turlin B, et al. Cytokines down-regulate expression of major cytochrome P-450 enzymes in adult human hepatocytes in primary culture. Mol Pharmacol. 1993;44(4):707–15.
Vahabzadeh M, Mohammadpour A. Effect of Diabetes Mellitus on the Metabolism of Drugs and Toxins. Journal of Clinical Toxicology. 2015;5(2).
Neyshaburinezhad N, Rouini M, Shirzad N, Esteghamati A, Nakhjavani M, Namazi S, et al. Evaluating the effect of type 2 diabetes mellitus on CYP450 enzymes and P-gp activities, before and after glycemic control: a protocol for a case-control pharmacokinetic study: evaluation of metabolism in diabetes mellitus. MethodsX. 2020;7:100853. https://doi.org/10.1016/j.mex.2020.100853.
Acknowledgements
This work was fully supported by a grant from National Institute for Medical Research Development of Iran (NIMAD) (grant no. 957596). The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Neyshaburinezhad, N., Seidabadi, M., Rouini, M. et al. Evaluation of hepatic CYP2D1 activity and hepatic clearance in type I and type II diabetic rat models, before and after treatment with insulin and metformin. DARU J Pharm Sci 28, 479–487 (2020). https://doi.org/10.1007/s40199-020-00350-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-020-00350-z