Abstract
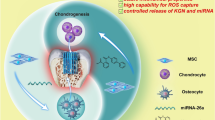
Conventional nanoporous hydrogels often lead to slow cartilage deposition by MSCs in 3D due to physical constraints and requirement for degradation. Our group has recently reported macroporous gelatin microribbon (μRB) hydrogels, which substantially accelerate MSC-based cartilage formation in vitro compared to conventional gelatin hydrogels. To facilitate translating the use of μRB-based scaffolds for supporting stem cell-based cartilage regeneration in vivo, there remains a need to develop a customize-designed drug delivery system that can be incorporated into μRB-based scaffolds. Towards this goal, here we report polydopamine-coated mesoporous silica nanoparticles (MSNs) that can be stably incorporated within the macroporous μRB scaffolds, and allow tunable release of transforming growth factor (TGF)-β3. We hypothesize that increasing concentration of polydopamine coating on MSNs will slow down TGF- β3 release, and TGF-β3 release from polydopamine-coated MSNs can enhance MSC-based cartilage formation in vitro and in vivo. We demonstrate that TGF-β3 released from MSNs enhance MSC-based cartilage regeneration in vitro to levels comparable to freshly added TGF-β3 in the medium, as shown by biochemical assays, mechanical testing, and histology. Furthermore, when implanted in vivo in a mouse subcutaneous model, only the group containing MSN-mediated TGF-β3 release supported continuous cartilage formation, whereas control group without MSN showed loss of cartilage matrix and undesirable endochondral ossification. The modular design of MSN-mediated drug delivery can be customized for delivering multiple drugs with individually optimized release kinetics, and may be applicable to enhance regeneration of other tissue types.




Similar content being viewed by others
References
Andrades, J. A., S. C. Motaung, P. Jiménez-Palomo, S. Claros, J. M. López-Puerta, J. Becerra, et al. Induction of superficial zone protein (SZP)/lubricin/PRG 4 in muscle-derived mesenchymal stem/progenitor cells by transforming growth factor-β1 and bone morphogenetic protein-7. Arthritis Res Therapy 14:R72, 2012.
Bai, Y.-X., Y.-F. Li, Y. Yang, and L.-X. Yi. Covalent immobilization of triacylglycerol lipase onto functionalized nanoscale SiO2 spheres. Process. Biochem. 41:770–777, 2006.
Barati, D., S. R. P. Shariati, S. Moeinzadeh, J. M. Melero-Martin, A. Khademhosseini, and E. Jabbari. Spatiotemporal release of BMP-2 and VEGF enhances osteogenic and vasculogenic differentiation of human mesenchymal stem cells and endothelial colony-forming cells co-encapsulated in a patterned hydrogel. J. Control. Release 223:126–136, 2016.
Bernsmann, F., V. Ball, F. Addiego, A. Ponche, M. Michel, J. J. D. A. Gracio, et al. Dopamine–melanin film deposition depends on the used oxidant and buffer solution. Langmuir 27:2819–2825, 2011.
Bharti, C., U. Nagaich, A. K. Pal, and N. Gulati. Mesoporous silica nanoparticles in target drug delivery system: a review. Int. J. Pharm. Investig. 5:124, 2015.
Cheng, W., J. Nie, L. Xu, C. Liang, Y. Peng, G. Liu, et al. pH-sensitive delivery vehicle based on folic acid-conjugated polydopamine-modified mesoporous silica nanoparticles for targeted cancer therapy. ACS Appl. Mater. Interfaces 9:18462–18473, 2017.
Chu, C. R., M. Szczodry, and S. Bruno. Animal models for cartilage regeneration and repair. Tissue Eng. B 16:105–115, 2010.
Conrad, B., L. H. Han, and F. Yang. Gelatin-based microribbon hydrogels accelerate cartilage formation by mesenchymal stem cells in 3D. Tissue Eng. A 24:1630–1641, 2018.
Erickson, I. Optimization and Translation of MSC-Based Hyaluronic Acid Hydrogels for Cartilage Repair. Pennsylvania: University of Pennsylvania, 2011.
Erickson, I. E., S. R. Kestle, K. H. Zellars, M. J. Farrell, M. Kim, J. A. Burdick, et al. High mesenchymal stem cell seeding densities in hyaluronic acid hydrogels produce engineered cartilage with native tissue properties. Acta Biomater. 8:3027–3034, 2012.
Ertan, A. B., P. Yılgor, B. Bayyurt, A. C. Çalıkoğlu, Ç. Kaspar, F. N. Kök, et al. Effect of double growth factor release on cartilage tissue engineering. J. Tissue Eng. Regener. Med. 7:149–160, 2013.
Fahy, N., M. Alini, and M. J. Stoddart. Mechanical stimulation of mesenchymal stem cells: implications for cartilage tissue engineering. J. Orthop. Res. 36:52–63, 2018.
Farrell, M. J., M. B. Fisher, A. H. Huang, J. I. Shin, K. M. Farrell, and R. L. Mauck. Functional properties of bone marrow-derived MSC-based engineered cartilage are unstable with very long-term in vitro culture. J Biomech. 47:2173–2182, 2014.
Fong, C.-Y., A. Subramanian, K. Gauthaman, J. Venugopal, A. Biswas, S. Ramakrishna, et al. Human umbilical cord Wharton’s jelly stem cells undergo enhanced chondrogenic differentiation when grown on nanofibrous scaffolds and in a sequential two-stage culture medium environment. Stem Cell Rev. Rep. 8:195–209, 2012.
Freyria, A.-M., and F. Mallein-Gerin. Chondrocytes or adult stem cells for cartilage repair: the indisputable role of growth factors. Injury 43:259–265, 2012.
Gegg, C., and F. Yang. Spatially patterned microribbon-based hydrogels induce zonally-organized cartilage regeneration by stem cells in 3D. Acta Biomater. 101:196–205, 2019.
Han, L.-H., B. Conrad, M. T. Chung, L. Deveza, X. Jiang, A. Wang, et al. Microribbon-based hydrogels accelerate stem cell-based bone regeneration in a mouse critical-size cranial defect model. J. Biomed. Mater. Res. A 104:1321, 2016.
He, Q., and J. Shi. Mesoporous silica nanoparticle based nano drug delivery systems: synthesis, controlled drug release and delivery, pharmacokinetics and biocompatibility. J. Mater. Chem. 21:5845–5855, 2011.
Karimi, T., D. Barati, O. Karaman, S. Moeinzadeh, and E. Jabbari. A developmentally inspired combined mechanical and biochemical signaling approach on zonal lineage commitment of mesenchymal stem cells in articular cartilage regeneration. Integr. Biol. 7:112–127, 2014.
Kim, T.-K., B. Sharma, C. Williams, M. Ruffner, A. Malik, E. G. McFarland, et al. Experimental model for cartilage tissue engineering to regenerate the zonal organization of articular cartilage. Osteoarthritis Cartil. 11:653–664, 2003.
Kisiday, J., M. Jin, B. Kurz, H. Hung, C. Semino, S. Zhang, et al. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc. Natl. Acad. Sci. USA 99:9996–10001, 2002.
Kondo, M., K. Yamaoka, K. Sonomoto, S. Fukuyo, K. Oshita, Y. Okada, et al. IL-17 inhibits chondrogenic differentiation of human mesenchymal stem cells. PLoS ONE 8:e79463, 2013.
Lee, H., S. M. Dellatore, W. M. Miller, and P. B. Messersmith. Mussel-inspired surface chemistry for multifunctional coatings. Science 318:426–430, 2007.
Lee, S., X. Tong, L.-H. Han, A. Behn, and F. Yang. Aligned microribbon-like hydrogels for guiding three-dimensional smooth muscle tissue regeneration. J. Biomed. Mater. Res. A 104:1064–1071, 2016.
Lissenberg-Thunnissen, S. N., D. J. de Gorter, C. F. Sier, and I. B. Schipper. Use and efficacy of bone morphogenetic proteins in fracture healing. Int. Orthop. 35:1271, 2011.
Liu, C.-Y., and C.-J. Huang. Functionalization of polydopamine via the aza-michael reaction for antimicrobial interfaces. Langmuir 32:5019–5028, 2016.
Madry, H., A. Rey-Rico, J. K. Venkatesan, B. Johnstone, and M. Cucchiarini. Transforming growth factor beta-releasing scaffolds for cartilage tissue engineering. Tissue Eng. B 20:106–125, 2013.
Motoyama, M., M. Deie, A. Kanaya, M. Nishimori, A. Miyamoto, S. Yanada, et al. In vitro cartilage formation using TGF-β-immobilized magnetic beads and mesenchymal stem cell-magnetic bead complexes under magnetic field conditions. J. Biomed. Mater. Res. A 92:196–204, 2010.
Mueller, M. B., M. Fischer, J. Zellner, A. Berner, T. Dienstknecht, L. Prantl, et al. Hypertrophy in mesenchymal stem cell chondrogenesis: effect of TGF-β isoforms and chondrogenic conditioning. Cells Tissues Organs 192:158–166, 2010.
Mueller, M. B., and R. S. Tuan. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 58:1377–1388, 2008.
Niger, C., K. E. Beazley, and M. Nurminskaya. Induction of chondrogenic differentiation in mesenchymal stem cells by TGF-beta cross-linked to collagen-PLLA [poly (L-lactic acid)] scaffold by transglutaminase 2. Biotechnol. Lett. 35:2193–2199, 2013.
O’Conor, C. J., N. Case, and F. Guilak. Mechanical regulation of chondrogenesis. Stem Cell Res. Therapy 4:61, 2013.
Park, J. S., K. Na, D. G. Woo, H. N. Yang, and K.-H. Park. Determination of dual delivery for stem cell differentiation using dexamethasone and TGF-β3 in/on polymeric microspheres. Biomaterials 30:4796–4805, 2009.
Pei, M., F. He, and G. Vunjak-Novakovic. Synovium-derived stem cell-based chondrogenesis. Differentiation 76:1044–1056, 2008.
Pelttari, K., A. Winter, E. Steck, K. Goetzke, T. Hennig, B. G. Ochs, et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 54:3254–3266, 2006.
Scotti, C., B. Tonnarelli, A. Papadimitropoulos, A. Scherberich, S. Schaeren, A. Schauerte, et al. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc. Natl Acad. Sci. 107:7251–7256, 2010.
Sellers, R. S., D. Peluso, and E. A. Morris. The effect of recombinant human bone morphogenetic protein-2 (rhBMP-2) on the healing of full-thickness defects of articular cartilage. JBJS 79:1452–1463, 1997.
Shintani, N., K. A. Siebenrock, and E. B. Hunziker. TGF-ss1 enhances the BMP-2-induced chondrogenesis of bovine synovial explants and arrests downstream differentiation at an early stage of hypertrophy. PLoS ONE 8:e53086, 2013.
Slowing, I. I., J. L. Vivero-Escoto, C. W. Wu, and V. S. Lin. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 60:1278–1288, 2008.
Spiller, K. L., S. A. Maher, and A. M. Lowman. Hydrogels for the repair of articular cartilage defects. Tissue Eng. B 17:281–299, 2011.
Steck, E., J. Fischer, H. Lorenz, T. Gotterbarm, M. Jung, and W. Richter. Mesenchymal stem cell differentiation in an experimental cartilage defect: restriction of hypertrophy to bone-close neocartilage. Stem Cells Dev. 18:969–978, 2009.
Tamanna, T., J. B. Bulitta, C. B. Landersdorfer, V. Cashin, and A. Yu. Stability and controlled antibiotic release from thin films embedded with antibiotic loaded mesoporous silica nanoparticles. RSC Adv. 5:107839–107846, 2015.
Tanaka, H., T. Sugita, Y. Yasunaga, S. Shimose, M. Deie, T. Kubo, et al. Efficiency of magnetic liposomal transforming growth factor-beta 1 in the repair of articular cartilage defects in a rabbit model. J. Biomed. Mater. Res. A 73:255–263, 2005.
Tang, F., L. Li, and D. Chen. Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv. Mater. 24:1504–1534, 2012.
Trippel, S. B. Growth factor actions on articular cartilage. J. Rheumatol. Suppl. 43:129–132, 1995.
Vinardell, T., E. J. Sheehy, C. T. Buckley, and D. J. Kelly. A comparison of the functionality and in vivo phenotypic stability of cartilaginous tissues engineered from different stem cell sources. Tissue Engineering. A 18:1161–1170, 2012.
Wang, T., J. H. Lai, and F. Yang. Effects of hydrogel stiffness and extracellular compositions on modulating cartilage regeneration by mixed populations of stem cells and chondrocytes in vivo. Tissue Eng. A 22:1348–1356, 2016.
Wang, Y., Q. Zhao, N. Han, L. Bai, J. Li, J. Liu, et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine 11:313–327, 2015.
Wei, Y., L. Gao, L. Wang, L. Shi, E. Wei, B. Zhou, et al. Polydopamine and peptide decorated doxorubicin-loaded mesoporous silica nanoparticles as a targeted drug delivery system for bladder cancer therapy. Drug Deliv. 24:681–691, 2017.
Winter, A., S. Breit, D. Parsch, K. Benz, E. Steck, H. Hauner, et al. Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 48:418–429, 2003.
Worster, A. A., B. D. Brower-Toland, L. A. Fortier, S. J. Bent, J. Williams, and A. J. Nixon. Chondrocytic differentiation of mesenchymal stem cells sequentially exposed to transforming growth factor-beta1 in monolayer and insulin-like growth factor-I in a three-dimensional matrix. J. Orthop. Res. 19:738–749, 2001.
Xia, P., X. Wang, Y. Qu, Q. Lin, K. Cheng, M. Gao, et al. TGF-β1-induced chondrogenesis of bone marrow mesenchymal stem cells is promoted by low-intensity pulsed ultrasound through the integrin-mTOR signaling pathway. Stem Cell Res. Therapy 8:281, 2017.
Acknowledgments
The authors would like to thank NIH R01DE024772, NIH R01AR074502, Stanford Coulter translational seed grant, and Stanford SPARK program for funding. C.G. would like to thank the Stanford Bio-X graduate fellowship for support. The authors would also like to thank Anthony Behn for technical assistance with mechanical testing.
Conflict of interest
No potential conflicts of interest exist.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Debra T. Auguste oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Barati, D., Gegg, C. & Yang, F. Nanoparticle-Mediated TGF-β Release from Microribbon-Based Hydrogels Accelerates Stem Cell-Based Cartilage Formation In Vivo. Ann Biomed Eng 48, 1971–1981 (2020). https://doi.org/10.1007/s10439-020-02522-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-020-02522-z