Abstract
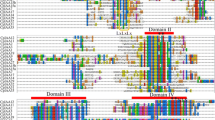
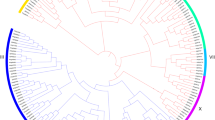
The “Nanguo” pear is a typically climacteric fruit and ethylene is the main factor controlling the ripening process of climacteric fruit. Ethylene biosynthesis has been studied clearly and ACC synthase (ACS) is the rate-limited enzyme. ACO (ACC oxidase) is another important enzyme in ethylene biosynthesis. By exploring the pear genome, we identified 13 ACS genes and 11 ACO genes, respectively, and their expression patterns in fruit and other organs were investigated. Among these genes, 11 ACS and 8ACO genes were expressed in pear fruits. What’s more, 4 ACS and 3ACO genes could be induced by Ethephon and inhibited by 1-MCP treatment. This study is the first time to explore ACS and ACO genes at genome-wide level and will provide new data for research on pear fruit ripening.




Similar content being viewed by others
References
Barry CS, Grierson LT (2000) The regulation of 1-aminocyclopropane-1-carboxylic acid synthase gene expression during the transition from system-1 to system-2 ethylene synthesis in tomato. Plant Physiol 123(3):979–986
Bouquin T, Lasserre E, Pradier J, Pech JC, Balague C (1997) Wound and ethylene induction of the acc oxidase melon gene cm-aco1 occurs via two direct and independent transduction pathways. Plant Mol Biol 35(6):1029–1035
Dandekar AM, Teo G, Defilippi BG, Uratsu SL, Passey AJ, Kader AA, Stow JR, Colgan RJ, James DJ (2004) Effect of down-regulation of ethylene biosynthesis on fruit flavor complex in apple fruit. Transgenic Res 13(4):373–384
Elsharkawy I, Kim WS, Jayasankar S, Svircev AM, Brown DCW (2008) Differential regulation of four members of the acc synthase gene family in plum. J Exp Bot 59(8):2009–2027
Gasic K, Hernandez A, Korban S (2004) RNA extraction from different apple tissues rich in polyphenols and polysaccharides for cDNA library construction. Plant Mol Biol Report 22:437a–437g
Guo H, Ecker JR (2004) The ethylene signaling pathway: new insights. Curr Opin Plant Biol 7:40–49
Huang G, Li T, Li X, Tan D, Jiang Z, Wei Y, Li J, Wang A (2014) Comparative transcriptome analysis of climacteric fruit of Chinese pear (Pyrus ussuriensis) reveals new insights into fruit ripening. PLoS One 9:e107562
Jung T, Lee JH, Cho MH, Kim WT (2010) Induction of 1-aminocyclopropane-1-carboxylate oxidase mRNA by ethylene in mung bean roots: possible involvement of Ca2+, and phosphoinositides in ethylene signalling. Plant Cell Environ 23(2):205–213
Kende H (1993) Ethylene biosynthesis. Annu Rev of Plant Physiol Mol Biol 44:283–307
Li T, Tan D, Yang X, Wang A (2013) Exploring the apple genome reveals six acc synthase genes expressed during fruit ripening. Sci Hortic 157:119–123
Li T, Xu Y, Zhang L, Ji Y, Tan D, Yuan H, Wang A (2017) The jasmonate-activated transcription factor MdMYC2 regulates ETHYLENE RESPONSE FACTOR and ethylene biosynthetic genes to promote ethylene biosynthesis during apple fruit ripening. Plant Cell 29:1316–1334
Lin Z, Zhong S, Grierson D (2009) Recent advances in ethylene research. J Exp Bot 60(12):3311–3336
Martel C, Vrebalov J, Tafelmeyer P, Giovannoni JJ (2011) The tomato MADS-box transcription factor RIPENING INHIBITOR interacts with promoters involved in numerous ripening processes in a COLORLESS NONRIPENING-dependent manner. Plant Physiol 157:1568–1579
Moon H, Callahan AM (2004) Developmental regulation of peach acc oxidase promoter-GUS fusions in transgenic tomato fruits. J Exp Bot 55(402):1519–1528
Nakatsuka A, Murachi S, Okunishi H, Shiomi S, Nakano R, Kubo Y, Inaba A (1998) Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase 1-aminocyclopropane-1-carboxylate oxidase and ethylene receptor genes in tomato fruit during development and ripening. Plant Physiol 118:1295–1305
Rodrigues-Pousada RA, Rycke RD, Dedonder A, Caeneghem WV, Engler G, Straeten MDVD (1993) The arabidopsis 1-aminocyclopropane-1-carboxylate synthase gene 1 is expressed during early development. Plant Cell 5(8):897–911
Schaffer RJ, Friel EN, Souleyre EJF, Bolitho K, Thodey K, LedgerS BJH, Ma JH, Nain B, Cohen D, Gleave AP, Crowhurst RN, Janssen BJ, Yao JL, Newcomb RD (2007) A genomics approach reveals that aroma production in apple is controlled by ethylene predominantly at the final step in each biosynthetic pathway. Plant Physiol 144(4):1899–1912
Tan D, Li T, Wang A (2013) Apple 1-aminocyclopropane-1-carboxylic acid synthase genes, mdacs1 and mdacs3a, are expressed in different systems of ethylene biosynthesis. Plant Mol Biol Report 31:204–209
Tsuchisaka A, Theologis A (2004) Heterodimeric interactions among the 1-amino-cyclopropane-1-carboxylate synthase polypeptides encoded by the Arabidopsis gene family. Proc Natl Acad Sci U S A 101(8):2275–2280
Yamamoto M, Miki T, Ishiki Y, Fujinami K, Sato T (1995) The synthesis of ethylene in melon fruit during the early stage of ripening. Plant Cell Physiol 36(4):591–596
Yang SF, Hoffman NE (1984) Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol 35:155–189
Yin XR, Allan AC, Chen KS, Ferguson IB (2010) Kiwifruit EIL and ERF genes involved in regulating fruit ripening. Plant Physiol 153(3):1280–1292
Yuan H, Zhang L, Jiang Z, Wang A (2017) Characterization of ripening-related puarp4 in pear (pyrus ussuriensis). J Plant Growth Regul 36:766–772
Funding
This work was supported by the National Natural Science Foundation of China (31801834).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Editor: Yong Eui Choi
Electronic supplementary material
Supplement Figure 1
The expression of PuACO6 in different tissues and in gDNA (JPG 95 kb)
Rights and permissions
About this article
Cite this article
Yuan, H., Yue, P., Bu, H. et al. Genome-wide analysis of ACO and ACS genes in pear (Pyrus ussuriensis). In Vitro Cell.Dev.Biol.-Plant 56, 193–199 (2020). https://doi.org/10.1007/s11627-019-10009-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-019-10009-3