Abstract
Elevated atmospheric CO2 may create greater methane (CH4) emissions from subarctic wetlands. To date such ecosystem feedbacks remain poorly understood, particularly in relation to how different wetland plant species will control such feedbacks. In this study we exposed plant-peat mesocosms planted with four Cyperaceae species to 400 and 800 ppm atmospheric CO2 concentrations and measured plant and peat properties as well as CH4 fluxes. Above ground biomass for plants grown at 800 ppm CO2 increased for E. angustifolium, Eriophorum vaginatum and Carex brunnescens, but the total biomass of C. acuta decreased relative to the ambient CO2 treatment. The plant species and elevated CO2 treatment affected both peat redox potential and pore water chemistry. There was no overall effect of the elevated CO2 on CH4 emissions, however, CH4 emissions were related to above ground biomass and redox potential, both of which were significantly altered by elevated CO2. Our study shows that species composition poses an important control on how wetland communities will respond to elevated CO2 and that plant mediated changes of peat biogeochemical processes, in response to elevated CO2 levels, may affect CH4 emissions from sub-arctic wetlands, but any such responses will differ among species.
Similar content being viewed by others
Introduction
Northern peatlands store ca. half of global soil carbon (C), much of which is held in permafrost areas (Tarnocai et al. 2009). Arctic and sub-arctic peatlands are responding rapidly to climate warming, threatening their C storage capacity (IPCC 2013). In parallel with rising temperatures, atmospheric CO2 levels have increased from pre-industrial levels of 280 ppm to 400 ppm with future atmospheric CO2 concentrations predicted to increase to between 426 ppm (RCP 2.6) and 936 ppm (RCP 8.5) over the next century (IPCC 2013). These changes in climate and atmospheric CO2 concentration may result in greater Net Primary Productivity (NPP) and decomposition rates which are both controls of greenhouse gas fluxes from ecosystems including wetlands (Curtis et al. 1989; Turetsky et al. 2014).
Wetlands release ca. 80% of methane (CH4) emission from natural sources, equating to a third of overall global emissions (Kirschke et al. 2013) with the largest CH4 atmospheric concentrations found north of 40o N (Steele et al. 1987). Indeed, the large area of wetlands at northern high latitudes are recognised as an important component of the global CH4 budget (Moore and Knowles 1990; Bridgham et al. 2013; Turetsky et al. 2014). Wetland CH4 emissions are determined by temperature, substrate and hydrology (Updegraff et al. 2001; Bridgham et al. 2013). In subarctic and arctic regions, these factors are strongly controlled by permafrost. Therefore future changes to permafrost are predicted to impact on CH4 emissions from high latitude regions (Christensen et al. 2004; IPCC 2013). For example, waterlogging of peatland soils as a result of permafrost thaw will likely increase CH4 emissions from arctic regions both in response to the anoxic conditions per se but also due to vegetation shifts from moss-lichen woody shrub tundra to wet sedge communities (Christensen et al. 2004; ACIA 2005; Bridgham et al. 2013).
Vegetation directly impacts CH4 emissions from peatlands (Joabsson et al. 1999; Heilman and Carlton 2001; Öquist and Svensson 2002; Ström et al. 2005; Bhullar et al. 2013a, b). The main controls of CH4 emissions in relation to vegetation are; (i) release of labile organic compounds in to the root zone which increase CH4 production; (ii) enhancing CH4 oxidation due to root oxygen emissions in to the peat and; (iii) passive transport of CH4 through plant tissues (Joabsson et al. 1999; Öquist and Svensson 2002). As most of the organic matter stored in arctic peatlands is recalcitrant and substrates for digestion by anaerobic bacteria are limiting (Bridgham et al. 2013; Sjogersten et al. 2016), input of labile photosynthates in the form of litter or root exudates are an important carbon source for methanogens (Torn and Chapin 1993; Ström et al. 2005). The diffusion of oxygen through aerenchyma from the atmosphere into the roots and leakage into the rhizosphere causes oxidation of CH4 to CO2 in the soil, substantially reducing net CH4 emissions (Fritz et al. 2011). The quality and quantity of plant litter and root exudate as well as root O2 inputs differs among wetland plant species, potentially creating species specific impacts on CH4 fluxes (Updegraff et al. 1995; Ström et al. 2005). Plant mediated transport of CH4 to the atmosphere can represent a major emission pathway but its contribution to net emissions varies among plant species as the rate of transport is strongly dependent on aerenchyma tissue (Kutzbach et al. 2004; Bhullar et al. 2013b). Indeed, while both Eriphorumn and Carex species can emit large amounts of CH4 through their tissues the proportion of CH4 emitted through plant tissues varies strongly among species (Bhullar et al. 2013b).
Elevated atmospheric CO2 can influence wetland CH4 production through its role in plant C assimilation and allocation. For example, greater root biomass in rice grown under elevated CO2 concentration has substantially increased CH4 emissions from paddy rice fields (Van Groenigen et al. 2011). Greater plant biomass and productivity in a range of wetland species in response to elevated CO2 have resulted in increased CH4 emissions from some wetland systems (Megonigal and Schlesinger 1997; Kao-Kniffin et al. 2011; Wang et al. 2013) while the growth of other species have not been affected resulting in no change in wetland CH4 fluxes (Angel et al. 2012). Such contrasting responses may, in part, be controlled by the plant species composition of different wetlands as elevated atmospheric CO2 concentrations influence plant activity including growth, photosynthetic rates and root exudate production, processes which also vary strongly among species (Lawlor and Mitchell 1991; Zak et al. 1993; Bellisario et al. 1999). These findings suggest that a more detailed understanding of how elevated CO2 impact different plant species is required in order to tease apart the controls that govern plant mediated impacts on CH4 emissions in response to elevated CO2. It is likely that if elevated atmospheric CO2 concentrations increase biomass, providing growth is not nutrient limited in the first place (Gordon et al. 2001), this will increase labile C inputs into the peat and potentially production of CH4. However, greater plant biomass may also increase transport of O2 to the rhizosphere and CH4 to the atmosphere (Joabsson et al. 1999; Wolf et al. 2007; Laanbroek 2010). Taken together, the understanding of impacts of elevated CO2 on arctic wetland CH4 emissions is limited at both the ecosystem and species level, creating large uncertainties in model predictions of the role of elevated CO2 on CH4 feedback mechanisms (Ringeval et al. 2011).
Arctic and sub-arctic peatlands are currently responding rapidly to climate warming, which has resulted in expansions of graminoid-dominated flooded areas (Prater et al. 2007; Åkerman and Johansson 2008). Such changes in the vegetation has lead to large increases in CH4 emissions (Christensen et al. 2004; Hodgkins et al. 2014). In subarctic and arctic wetlands, graminoids (e.g. Eriophorum sp. and Carex sp.) are known to directly impact CH4 emissions both by transport of CH4 from the rhizosphere to the atmosphere (Bhullar et al. 2013b; Turetsky et al. 2014) and by impacting CH4 production and oxidation in the rhizosphere (Ström et al. 2005; Koelbener et al. 2010; Fritz et al. 2011; Ström et al. 2012). However, these effects are species specific (Christensen et al. 2004; Ström et al. 2005; Prater et al. 2007; Hodgkins et al. 2014). To explore how variation in graminoid species composition and elevated atmospheric CO2 impacts CH4 emissions we established a controlled environment experiment exposing peat mesocosms planted with either C. acuta, C. brunnescens, E. vaginatum or E. angustifolium to elevated CO2 and measured how this affected plant growth, peat physicochemical properties and CH4 fluxes. This experiment was used to test the hypothesis that: Elevated atmospheric CO2 will increase productivity of Carex and Eriophorum species and subsequently stimulate CH4 emissions due to increased root inputs of labile substrates for methanogens, with the largest effect of elevated CO2 on CH4 emissions found in peats planted with C. acuta (the highest biomass species).
Methods
Site Description
The study site is a subarctic peatland located on the southern edge of Lake Torneträsk in Northern Sweden (68° 21′ 30.96“ N 18° 46’ 56.064” E). The mean annual precipitation is 310 mm, more than 40% of this occurs during summer. Mean annual temperature is 0.7 °C, with a July average of 11 °C (1913–2000 average, Kohler et al. 2006). The site is a palsa mire complex, a common peatland type in the region. The area is made up of two distinct communities of vegetation (Sjogersten et al. 2016). The raised, mesic area is dominated by dwarf shrubs (Betula nana, Empetrum nigrum and Vaccinium uliginosum). The active layer depth in these hummocks is 30 ± 0.9 cm in summer. In the flooded areas there are three dominant Cyperaceae species: C. acuta, E. angustifolium and E. vaginatum as well as the less common C. brunnescens (Nilsson 1991; Mossberg and Stenberg 2008). On average, (n = 5) C. acuta grew in locations with an active layer depth of 119 ± 21 cm below the peat surface, E. angustifolium at 122 ± 12 cm and E. vaginatum and C. brunnescens at 95 ± 21 cm. The water level depth in the flooded areas varied, averaging +34 ± 7 cm where C. acuta was found, + 30 ± 3 cm for E. angustifolium and + 15 ± 2 cm for E. vaginatum and C. brunnescens, note that positive values means that the peat surface was submerged. The peat pH at 5 cm depth was 4.3 ± 0.04; with conductivity of 66 ± 30.8 μS; extractable PO43− of 3.9 ± 1 μg g−1 and extractable NH4+ of 0.12 ± 0.02 μg g−1. Peat pH and conductivity was determined following mixing peat with DI water in a 1:2 ratio by volume and analysis on a dual pH and conductivity analyser. Extractable PO43− and NH4+ was determined using standard colourimetrically methods following a K2SO4 extraction of 5 g of fresh weight soil.
Experimental Design and Analysis
Growth Room Experiments
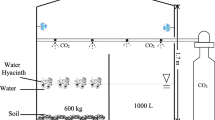
Growth room experiments were established using two walk-in growth rooms (Unigrow, UK) which had fixed atmospheric CO2 concentrations of 400 ppm and 800 ppm. Mesocosms planted with either C. acuta, C. brunnescens, E. angustifolium or E. vaginatum were established with peat and plant material collected from the field site. The degree of replication per treatment was; n = 10 for C. acuta and E. vaginatum, n = 6 for E. augustifolium, and n = 5 for C. brunnescens, this resulted in a total number of 42 mesocosms. Peat samples were collected as several 20 × 20 × 20 cm blocks taken ca. 30 cm below the peat surface from submerged areas free of vegetation with a water table depth of ca. 30 cm. The recovered plant and soil samples were transported, separated and transplanted into separate water-tight one litre pots ensuring good contact between plant roots and the peat. Care was taken that peat did not dry out prior to the experiment started, however, some oxygenation of the peat will have occurred. For C. acuta, C. brunnescens, E. angustifolium individual shoots were transplanted in to the peat while E. vaginatum was planted as small tussocks reflecting the plants growth form in the field. As the starting biomass differed among pots the mesocosms were grouped as pairs according to biomass and then randomly allocated to a CO2 treatment. The volume of peat in the pots were ca. one litre, water levels were adjusted (using tap water) to 2–3 cm above the peat surface throughout the experiment. This water level is shallower than those in the field but where used to reduce ebullition and bubble formation on the inside edges of the mesocosm which were deemed to be a risk associated with using deeper water levels. The conditions used in the growth chambers was a day length of 16 h, day/night temperature was 21/15 °C, reflecting the conditions found at the sites during warm summers to stimulate growth and microbial activity. The relatively high temperatures compared to the average air temperature during the field sampling period of 13.3 °C. Daytime light levels were constant at 400 μmol m−2 s−1 and day/night humidity was 65/75%. The mesocosms were equilibrated for ca. 1 month before the measurements started to allow the system to settle from the disturbance and any remaining oxygen to be used up so that the experiment took place under anoxic conditions.
Two types of head space chamber were used for the gas sampling. A taller chamber (15 cm diameter × 100 cm height, 17.7 l volume) was used for the mesocosms with C. acuta and E. angustifolium and a smaller chamber (15 cm diameter × 25 cm height, 4.4 l volume) was used for the shorter E. vaginatum and C. brunnescens.
To define individual plant-mediated methane-controlling mechanisms over the experimental period, CH4 flux, redox, and plant extension growth measurements were measured fortnightly during daytime conditions. These measurements were taken at five time points over a 10 week period between January and April 2015. Methane fluxes were determined using static headspace chambers (Denmead 2008) with samples collected over 20 min. Air in the chambers was circulated using small computer fans. The air samples were stored in 12 ml exetainers (Labco, Lampeter, UK). CH4 concentrations were determined by gas chromatography (GC-2014, Shimadzu UK LTD, Milton Keynes, UK) using a single injection system with a 1 mL sample loop that passed the gas sample using H2 as carrier, the flow rate was 30 ml min−1. The oven temperature was 40 °C and the column was a molecular sieve, the injector temperature was 80 °C. Flame ionization (detector temperature was 250 °C) detectors were used to measure CH4. The analytical error was ca. 5%. Methane fluxes were calculated using the ideal gas law (e.g. Mangalassery et al. 2014) and were expressed as both per unit area and peat dry weight.
Peat redox potential was measured in three points in each pot using a redox probe (General Purpose ORP Electrode, Van London Phoenix, Randburg, South Africa) connected to a millivolt pH meter. To assess plant growth, three leaves of each individual plant were labelled and extension growth recorded. At the end of the experiment, pore-water samples were extracted from each mesocosm using rhizon samplers (Rhizosphere Research Products, Wageningen, Netherlands). In the soil solution we determined the E4:E6 ratio, which is an indices of the humification capacity of dissolved organic carbon in the solution, using a spectrophotometer (Cecil CE1011 1000 series) set at 465 nm and 665 nm (Worrall et al. 2002). TOC-TN analysis (Shimadzu TOC-V CPH; TNM-1) was used to measure the total dissolved organic carbon (TOC) and total dissolved nitrogen (TN) content of the water with the ratio of TOC:TN reflecting the lability of carbon in the pore water (Kokfelt et al. 2009). Above and below ground biomass of plant samples was separated, roots were picked from the peat using tweezers and then washed. The biomass samples and the remaining peat were dried at 60 °C for 72 h then weighed to calculate total above and below ground biomass and peat dry weight.
Data Analysis
Data analysis was carried out using GenStat (15th Edition). Treatment effects on plant extension growth and biomass production, pore water chemistry and CH4 fluxes was assessed using linear mixed models. In the model we used the CO2 treatment, species treatment and time as fixed factors while individual mesocosms were used as the random factor. Statistics reported are the F-value, which is the ratio for between group variance and within group variance, numerator (i.e. fixed) degrees of freedom and denominator (i.e. residual) degrees of freedom, the P value indicating significance when <0.05. When required, data were transformed to meet the normality assumption. Linear regression was used to determine relationships between variables (e.g. CH4 fluxes, biomass, pore water chemistry).
Results
Extension growth differed among species with the greatest extension growth found for C. acuta (species effect: F3, 53 = 11.32, P < 0.001), the growth rates of the other three species was in the same range (e.g. 24 ± 2, 12 ± 2.8, 13 ± 2.6, 11 ± 2.0 mm week−1, respectively for C. acuta, C. brunnescens, E. angustifolium, E. vaginatum at the start of the experiment). The elevated CO2 treatment caused a significant overall reduction in extension growth (CO2 treatment effect: F1, 53 = 12.11; P < 0.001; Fig. 1) with a particularly strong negative impact for C. acuta. At the end of the experiment, above ground biomass differed significantly among species with the lowest biomass found for C. brunnescens (F 3,52 = 8.34, P < 0.001; Fig. 2a). The elevated atmospheric CO2 levels tended to increase above ground biomass in E. angustifolium, E. vaginatum and C. brunnescens but decreased above ground biomass in C. acuta (near significant species × CO2 treatment interaction: F 1,52 = 3.58, P = 0.064; Fig. 2a). Below ground biomass differed among species (Fig. 2b; F3,52 = 8.31, P < 0.001); furthermore the CO2 treatment affected belowground biomass differently among the four species (species × CO2 treatment interaction: F3,52 = 2.58, P = 0.063) as below ground biomass declined in the elevated CO2 treatment relative to ambient CO2 for E. angustifolium and C. acuta. As a result of the contrasting above and below ground biomass responses among species to the CO2 treatment, the response of the shoot:root ratios to elevated CO2 differed among the species (Fig. 2c; species × CO2 treatment interaction: F3,52 = 6.45, P < 0.001). Specifically the shoot:root ratio increased for all species apart from C. brunnescens.
The plant species treatment strongly affected CH4 fluxes with the greatest emissions from E. angustifolium and the lowest emissions from C. brunnescens (Fig. 3; F3, 52 = 5.57, P = 0.002). In contrast, the CO2 treatment did not affect the CH4 fluxes (F3, 52 = 0.79, P = 0.39).
The CO2 and species treatment affected soil properties. Specifically, redox potential was consistantly lower in the 800 ppm treatment (F1, 53 = 3.39, P = 0.07, Fig. 4). Redox also differed among the plant species treatments (F3, 53 = 27.01, P < 0.001) with the two Carex species having the highest redox potentials. The redox potential was negatively related to above ground plant biomass (Fig. 5a; F1, 7 = 4.27, P = 0.08). The elevated CO2 treatment had a contrasting effect on total dissolved organic carbon (TOC) for the different plant species ((species × CO2 treatment interaction, F3, 52 = 2.82, P = 0.048), Fig. 6a). Specifically, E. vaginatum and C. acuta differed in their response to elevated CO2, with pore water in the 800 ppm treatment exhibiting 0.9 mg L−1 more organic carbon in E. vaginatum but 1.5 mg L−1 less in C. acuta when compared to ambient CO2 conditions. In contrast, TOC levels for E. angustifolium and C. brunnescens were not affected by the CO2 treatment.
Means with standard error for (a) Total Organic Carbon (TOC); b Total Nitrogen (TN); c TOC:TN ratio and; d E4:E6 ratio in pore water samples for four Cyperaceae species under atmospheric CO2 conditions of 400 and 800 ppm. S.E.D for the Species and Species × CO2 Treatment effects are shown in the figure
Total dissolved nitrogen (TN) (Fig. 6b) was not significantly influenced by treatment or species effects. The TOC:TN ratio differed among species and was highest in E. vaginatum (F3, 52 = 7.91, P < 0.001, Fig. 6c) out of the planted treatments but was not affected by the CO2 treatment. Pore water in the two species of Carex display the highest E4:E6 ratio (i.e. relatively more low molecular weight compounds) (F3, 52 = 6.05, P = 0.001, Fig. 6d) but again there was no significant difference between the CO2 treatments.
Although the CH4 fluxes were not directly related to plant biomass, redox potential, which was influenced by the above ground biomass, was negatively related to CH4 fluxes (Fig. 5b; F1, 7 = 5.75, P = 0.05). In contrast, the pore water chemistry was not related to the CH4 fluxes.
Discussion
The increase in above ground biomass for three of the four study species in response to the elevated CO2 treatment (Fig. 2a) supported the hypothesis, which predicted greater biomass production under high CO2 conditions. The different responses of above ground biomass of the study species to increased CO2 concentrations (Fig. 2a) suggest that wetland plant species will respond in contrasting ways to rising CO2 concentrations. Previous studies support the notion of species specific responses to elevated CO2. For example, following two years of experimental CO2 treatments (ambient +340 ppm) different biomass responses was reported for Schoenoplectus americanus and Spartina patens in a salt marsh ecosystem (Langley et al. 2013). This was also the case for above ground biomass of Typha species (T. angustifolia, T. glauca and T. latifolia) exposed to 350–390 (control) to 550–600 ppm (treatment) CO2 (Sullivan et al. 2010). The lower extension growth under elevated CO2 indicates that greater above ground biomass is likely due to increased tillering and number of leaves. However, of the Typha species investigated by Sullivan et al. (2010), all increased their below ground biomass in response to the elevated CO2 treatment. This differs to our study in which above ground biomass of E. angustifolium, E. vaginatum and C. brunnescens was higher in the elevated CO2 treatment while below ground biomass only increased for two of the study species (E.vaginatum and C. brunnescens (Fig. 2b)). No below ground responses for Schoenoplectus americanus and Spartina patens was also reported by Langley et al. (2013) after two years exposure to elevated CO2. Species specific responses to atmospheric CO2 are well known, with fundamental differences in stomatal numbers and size being observed (Woodward et al. 2002; Lomax et al. 2014), which can then influence physiology and ultimately impact on biomass.
This study has demonstrated that different plant species control the amount and quality of substrate found in the pore water, with elevated atmospheric CO2 influencing TOC concentrations in planted treatments (Fig. 6a), largely reflecting trends in biomass (Fig. 2). This correlates with data from temperate salt marshes exposed to elevated CO2 (Marsh et al. 2005; Keller et al. 2009). In addition, the trend of lower root biomass, lower TOC and TN concentrations and lower CH4 emissions in the 800 ppm C. acuta treatment suggests a link among these parameters. Indeed, contrasting above/below ground carbon allocation and quantity and quality of root exudates (e.g. Ström et al. 2005; Koelbener et al. 2010; Ström et al. 2012) may drive some of the species specific responses to elevated CO2 and the subsequent impact on CH4 emissions. The contrasting porewater chemistry with regard to the E4:E6 and TOC:TN ratios (Fig. 6 c and d) suggests that species composition alters rhizospheric organic matter inputs, likely due to differences in the concentration and lability of root exudates, with implications for CH4 fluxes (King et al. 2002; Ström et al. 2005; Dorodnikov et al. 2011). Furthermore, root exudates have been found to enhance degradation of older recalcitrant soil organic matter which may further increase substrate availability for CH4 production (Basiliko et al. 2012). The study was too short to measure how elevated CO2 may alter litter chemistry, however, larger biomass as a result of elevated CO2 (namely in C. brunnescens and E. vaginatum, Fig. 2) is likely to increase inputs from freshly produced litter which may also increase CH4 production (Curtis et al. 1990).
The reduction in redox potential under the elevated CO2 treatment (Fig. 4), together with the negative relationship between above ground biomass and soil redox potential (Fig. 5a), suggests that plant-mediated shifts in soil redox potential in response to elevated CO2 may impact soil processes. As soil redox potential is a strong driver of CH4 production and oxidation (Fritz et al. 2011) such changes may impact CH4 fluxes. Effects of elevated CO2 on soil redox potential were also demonstrated in mesocosms with Phragmites australis grown under ambient and elevated CO2 (+330 ppm CO2) (Mozdzer and Megonigal 2013). We speculate that the lowering of the redox potential is caused by greater release of organic material into the rhizosphere contributing to microbial respiration and depletion of electron donors (Yavitt and Seidman-Zager 2006; Laanbroek 2010). Our findings and those of Mozdzer and Megonigal (2013) contrast with those of Wolf et al. (2007) who demonstrated higher soil redox potentials in mesocosms planted with Scirpus olneyi due to greater root O2 inputs reflecting greater root biomass in the elevated CO2 treatment. The differing impact of plant species on the soil redox conditions, possibly in response to different levels of root exudation among species and/or differences in gas exchange via aerenchyma, suggests that plant species composition is a key control of the redox environment and by extension CH4 production and oxidation (Bridgham et al. 2013).
In contrast to our hypothesis, which predicted increased CH4 production in the elevated CO2 treatment, there was no significant effect of the elevated CO2 treatment on CH4 fluxes (Fig. 3b). However, the negative relationship between above ground biomass and redox potential, and redox potential and CH4 fluxes, demonstrates the important role of the vegetation as a control of redox and CH4 fluxes (Fig. 5). Our lack of direct responses of CH4 fluxes to elevated CO2 contrasts with studies on Taxodium distichum and Orontium aquaticum mesocosms exposed to an experimental increase in CO2 levels from 350 to 700 ppm (Vann and Megonigal 2003) and mesocosms planted with Typha angustifolia when CO2 levels were increased from 380 to 700 ppm (Kao-Kniffin et al. 2011), where increased root growth under elevated CO2 translated in to greater CH4 emissions. However, limited or no impact of elevated CO2 on CH4 emissions was found in two sedge dominated salt marsh communities (Marsh et al. 2005) illustrating that CH4 responses to elevated CO2 may vary strongly among species and ecosystems depending on the physiological responses of the plant species to elevated CO2. Furthermore, limited responses to elevated CO2 by some species may be linked to nutrient limitation (Mozdzer and Megonigal 2013).
In conclusion, we have demonstrated that elevated atmospheric CO2 increased above ground biomass production in E. vaginatum, E. angustifolium and C. brunnescens but not in C. acuta. Our study suggests links between plant biomass, soil redox potential and CH4 production but no direct impact of elevated CO2 on CH4 emissions. Our study demonstrates the importance for improved mechanistic understanding of how wetland plants species respond to elevated CO2 before assumptions can be made with regard to impacts on elevated CO2 on CH4 emissions from wetlands.
References
ACIA (2004) Impacts of a warming arctic: Arctic climate impact assessment. ACIA Overview report. Cambridge University Press, Cambridge pp 140
Åkerman HJ, Johansson M (2008) Thawing permafrost and thicker active layers in sub-Arctic Sweden. Permafrost Periglac 19(3):279–292
Angel R, Kammann C, Claus P, Conrad R (2012) Effect of long-term free-air CO2 enrichment on the diversity and activity of soil methanogens in a periodically waterlogged grassland. Soil Biology and Biochemistry 51:96–103
Basiliko N, Stewart H, Roulet NT, Moore TR (2012) Do root exudates enhance peat decomposition? Geomicrobiology Journal 29(4):374–378
Bellisario LM, Bubier JL, Moore TR, Chanton JP (1999) Controls on CH4 emissions from a northern peatland. Global Biogeochemical Cycles 13(1):81–91
Bhullar GS, Iravani M, Edwards PJ, Venterink HO (2013a) Methane transport and emissions from soil as affected by water table and vascular plants. BMC Ecology 13:32
Bhullar GS, Edwards PJ, Venterink HO (2013b) Variation in the plant-mediated methane transport and its importance for methane emission from intact wetland peat mesocosms. Plant Ecology, rts045
Bridgham SD, Cadillo-Quiroz H, Keller JK, Zhuang Q (2013) Methane emissions from wetlands: biogeochemical, microbial, and modeling perspectives from local to global scales. Global Change Biology 19(5):1325–1346
Christensen TR, Johansson T, Åkerman HJ, Mastepanov M, Malmer N, Friborg T, Svensson BH (2004) Thawing sub-arctic permafrost: Effects on vegetation and methane emissions. Geophysical Research Letters 31(4)
Curtis PS, Drake BG, Leadley PW, Arp WJ, Whigham DF (1989) Growth and senescence in plant communities exposed to elevated CO2 concentrations on an estuarine marsh. Oecologia 78(1):20–26
Curtis PS, Balduman LM, Drake BG, Whigham DF (1990) Elevated atmospheric CO2 effects on belowground processes in C3 and C4 estuarine marsh communities. Ecology 2001-2006
Denmead (2008) Approaches to measuring fluxes of methane and nitrous oxide between land scapes and the atmosphere. Plant and Soil 309:5–24
Dorodnikov M, Knorr KH, Kuzyakov Y, Wilmking M (2011) Plant-mediated CH4 transport and contribution of photosynthates to methanogenesis at a boreal mire: a 14C pulse-labeling study. Biogeosciences 8(8):2365–2375
Fritz C, Pancotto VA, Elzenga J, Visser EJ, Grootjans AP, Pol A, Smolders AJ (2011) Zero methane emission bogs: extreme rhizosphere oxygenation by cushion plants in Patagonia. The New Phytologist 190(2):398–408
Gordon C, Wynn JM, Woodin SJ (2001) Impacts of increased nitrogen supply on high Arctic heath: the importance of bryophytes and phosphorus availability. New Phytologist 149(3):461–471
Heilman MA, Carlton RG (2001) Methane oxidation associated with submersed vascular macrophytes and its impact on plant diffusive methane flux. Biogeochemistry 52(2):207–224
Hodgkins SB, Tfaily MM, McCalley CK, Logan TA, Crill PM, Saleska SR, Rich VI, Chanton JP (2014) Changes in peat chemistry associated with permafrost thaw increase greenhouse gas production. Proceedings of the National Academy of Sciences of the United States of America 111(16):5819–5824
IPCC (2013) Climate Change 2013: The Physical Science Basis. Contribution of working group I to the fifth assessment report of the Intergovernmental Panel on Climate Change. In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Cambridge University Press, Cambridge, pp 1535. https://doi.org/10.1017/CBO9781107415324
Joabsson A, Christensen T, Wallén B (1999) Vascular plant controls on methane emissions from northern peatforming wetlands. Trends Ecol Evol 14(10):385–388
Kao-Kniffin J, Freyre DS, Balser TC (2011) Increased methane emissions from an invasive wetland plant under elevated carbon dioxide levels. Applied Soil Ecology 48(3):309–312
Keller J, Wolf AA, Weisenhorn PB, Drake AEBG, Megonigal JP (2009) Elevated CO2 affects porewater chemistry in a brackish Marsh. Biogeochemistry 96:101–117. https://doi.org/10.1007/s10533-009-9347-3
King JY, Reeburgh WS, Thieler KK, Kling GW, Loya WM, Johnson LC, Nadelhoffer KJ (2002) Pulse-labelling studies of carbon cycling in Arctic tundra ecosystems: The contribution of photosynthates to methane emission. Global Biogeochemical Cycles 16(4):10-1–10-8
Kirschke S, Bousquet P, Ciais P, et al. (2013) Three decades of global methane sources and sinks. Nature Geoscience. https://doi.org/10.1038/ngeo1955
Koelbener A, Ström L, Edwards PJ, Venterink HO (2010) Plant species from mesotrophic wetlands cause relatively high methane emissions from peat soil. Plant and Soil 326(1–2):147–158
Kohler J, Brandt O, Johansson M, Callaghan T (2006) A long-term Arctic snow depth record from Abisko, northern Sweden, 1913–2004. Polar Research 25(2):91–113
Kokfelt U, Rosén P, Schoning K, Christensen TR, Förster J, Karlsson J, Reuss N, Rundgren M, Callaghan TV, Jonasson C, Hammarlund D (2009) Ecosystem responses to increased precipitation and permafrost decay in subarctic Sweden inferred from peat and lake sediments. Global Biogeochemical Cycles 15(7):1652–1663
Kutzbach L, Wagner D, Pfeiffer E-V (2004) Effect of micro-relief and vegetation on methane emission from wet polygonal tundra, Lena delta, Morthern Siberia. Biogeochemistry 69:341–362
Laanbroek HJ (2010) Methane emission from natural wetlands: interplay between emergent macrophytes and soil microbial processes. A mini-review. Annals of Botany 105:141–153
Langley JA, Mozdzer TJ, Shepard KA, Hagerty SB, Megonigal JP (2013) Tidal marsh plant responses to elevated CO2, nitrogen fertilization, and sea level rise. Global Change Biology 19(5):1495–1503
Lawlor DW, Mitchell RAC (1991) The effects of increasing CO2 on crop photosynthesis and productivity: a review of field studies. Plant, Cell & Environment 14(8):807–818
Lomax BH, Hilton J, Bateman RM, Upchurch GR, Lake JA, Leitch IJ, Cromwell A, Knight CA (2014) Reconstructing relative genome size of vascular plants through geological time. The New Phytologist 201:636–644
Mangalassery S, Sjögersten S, Sparkes DL, Sturrock CJ, Craigon J, Mooney SJ (2014) To what extent can zero tillage lead to a reduction in greenhouse gas emissions from temperate soils? Scientific Reports 4
Marsh AS, Rasse DP, Drake BG, Megonigal JP (2005) Effect of elevated CO2 on carbon pools and fluxes in a brackish marsh. Estuaries 28(5):694–704
Megonigal JP, Schlesinger WH (1997) Enhanced CH4 emission from a wetland soil exposed to elevated CO2. Biogeochemistry 37(1):77–88
Moore TR, Knowles R (1990) Methane emissions from fen, bog and swamp peatlands in Quebec. Biogeochemistry 11(1):45–61
Mossberg B, Stenberg L (2008) Fjällflora. Sverige, Finaland, Norge and Svalbard. Wahlstrom & Widstrand, Värnamo
Mozdzer TJ, Megonigal JP (2013) Increased methane emissions by an introduced Phragmites australis lineage under global change. Wetlands 33(4):609–615
Nilsson Ö (1991) Nordisk fjällflora. Bonniers
Öquist MG, and Svensson, BH (2002) Vascular plants as regulators of methane emissions from a subarctic mire ecosystem. Journal of Geophysical Research-Atmospheres (1984–2012), 107(D21), ACL 10-1 – 10-10
Prater JL, Chanton JP, and Whiting GJ (2007) Variation in methane production pathways associated with permafrost decomposition in collapse scar bogs of Alberta, Canada. Global Biogeochemical Cycles 21(4)
Ringeval B, Friedlingstein P, Koven C, Ciais P, Noblet-Ducoudré ND, Decharme B, Cadule P (2011) Climate-CH 4 feedback from wetlands and its interaction with the climate-CO 2 feedback. Biogeosciences 8(8):2137–2157
Sjogersten S, Caul S, Daniell TJ, O’Sullivan OS, Stapleton CS, Titman JJ (2016) Organic matter chemistry controls greenhouse gas emissions from permafrost peatlands. Soil Biology and Biochemistry 98:42–53
Steele LP, Fraser PJ, Rasmussen RA, Khalil MAK, Conway TJ, Crawford AJ, Thoning KW (1987) The global distribution of methane in the troposphere. In Scientific Application of Baseline Observations of Atmospheric Composition (SABOAC), 417–463. Springer Netherlands
Ström L, Mastepanov M, Christensen TR (2005) Species-specific effects of vascular plants on carbon turnover and methane emissions from wetlands. Biogeochemistry 75(1):65–82
Ström L, Tagesson T, Mastepanov M, Christensen TR (2012) Presence of Eriophorum scheuchzeri enhances substrate availability and methane emission in an Arctic wetland. Soil Biology and Biochemistry 45:61–70
Sullivan L, Wildova R, Goldberg D, Vogel C (2010) Growth of three cattail (Typha) taxa in response to elevated CO2. Plant Ecology 207(1):121–129
Tarnocai C, Canadell JG, Schuur EAG, Kuhry P, Mazhitova G, and Zimov S (2009) Soil organic carbon pools in the northern circumpolar permafrost region. Global Biogeochemical Cycles 23(2)
Torn MS, Chapin FS (1993) Environmental and biotic controls over methane flux from arctic tundra. Chemosphere 26(1):357–368
Turetsky M, Kotowska A, Bubier J et al (2014) A synthesis of methane emissions from 71 northern, temperate, and subtropical wetlands. Global Change Biology 20:2183–2197
Updegraff K, Pastor J, Bridgham SD, Johnston CA (1995) Environmental and substrate controls over carbon and nitrogen mineralization in northern wetlands. Ecological Applications 5(1):151–163
Updegraff K, Bridgham SD, Pastor J, Weishampel P, Harth C (2001) Response of CO2 and CH4 emissions from peatlands to warming and water table manipulation. Ecological Applications 11(2):311–326
Van Groenigen KJ, Osenberg CW, Hungate BA (2011) Increased soil emissions of potent greenhouse gases under increased atmospheric CO2. Nature 475:214–216
Vann C, Megonigal JP (2003) Elevated CO2 and water depth regulation of methane emissions: comparison of woody and non-woody wetland plant species. Biogeochemistry 63:117–134
Wang JM, Murphy JG, Geddes JA, Winsborough CL, Basiliko N, Thomas SC (2013) Methane fluxes measured by eddy covariance and static chamber techniques at a temperate forest in Central Ontario, Canada. Biogeosciences 10(6):4371–4382
Wolf AA, Drake BG, Erickson JE, Megonigal JP (2007) An oxygen-mediated positive feedback between elevated carbon dioxide and soil organic matter decomposition in a simulated anaerobic wetland. Global Change Biology 13(9):2036–2044
Woodward FI, Lake JA, Quick WP (2002) Stomatal development and CO2: ecological consequences. The New Phytologist 153:477–484
Worrall F, Burt TP, Jaeban RY, Warburton J, Shedden R (2002) Release of dissolved organic carbon from upland peat. Hydrological Processes 16(17):3487–3504
Yavitt JB, Seidman-Zager M (2006) Methanogenic conditions in northern peat soils. Geomicrobiology Journal 23(2):119–127
Zak DR, Pregitzer KS, Curtis PS, Teeri JA, Fogel R, Randlett DL (1993) Elevated atmospheric CO2 and feedback between carbon and nitrogen cycles. Plant and Soil 151(1):105–117
Acknowledgements
We are grateful to James Verran and John Corrie for laboratory for technical support. The project was funded by the University of Nottingham.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bridgman, M.J., Lomax, B.H. & Sjögersten, S. Impacts of Elevated Atmospheric CO2 and Plant Species Composition on Methane Emissions from Subarctic Wetlands. Wetlands 40, 609–618 (2020). https://doi.org/10.1007/s13157-019-01203-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-019-01203-5