Abstract
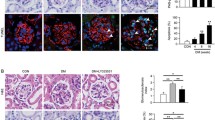
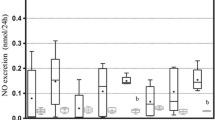
Previous studies in our laboratory have suggested that P2X7 could contribute to the progression of diabetic nephropathy and modulated klotho expression. The aim of this study was to investigate if P2X7 receptor is related to the expression of klotho in the onset of diabetic nephropathy in rats. Seven-week-old male Wistar rats weighing 210 g were all uninephrectomized; two-third of the animals were induced to diabetes with 60 mg/kg streptozotocin i.v., and one-third received its vehicle (control rats). At 4th day of the fifth week of the protocol, half of the diabetic rats received a small interfering RNA targeting for P2X7 mRNA, and the other half received its vehicle. Euthanasia was made at the eighth week. Diabetic animals reproduced all classic symptoms of the disease; besides, they showed reduced renal function and low NO bioavailability; also, SOD1, SOD2, and catalase were increased, probably due to the oxidative stress which was elevated in this situation. Metabolic data of diabetic rats did not change by silencing P2X7 receptor. For the other hand, silencing P2X7 was able to contribute to balance oxidative and nitrosative profile, ultimately improving the renal function and increasing plasma and membrane forms of klotho. These findings suggest that the management of P2X7 receptor can benefit the kidneys with diabetic nephropathy. Further studies are needed to show the therapeutic potential of this receptor inhibition to provide a better quality of life for the diabetic patient.






Similar content being viewed by others
References
Molitch ME, DeFronzo RA, Franz MJ (2004) Nephropathy in diabetes. Diabetes Care 27(suppl 1):s79–s83. https://doi.org/10.2337/diacare.27.2007.S79
Costa G, Pereira T, Neto AM, Cristovao AJ, Ambrosio AF, Santos PF (2009) High glucose changes extracellular adenosine triphosphate levels in rat retinal cultures. J Neurosci Res 87(6):1375–1380. https://doi.org/10.1002/jnr.21956
Burnstock G (1978) A basis for distinguishing two types of purinergic receptor. In: Straub RW, Bolis CL (eds) Cell membrane receptors for drugs and hormones: a multidisciplinary approach, vol 1. Raven Press, New York, pp 107–118. https://doi.org/10.1016/0968-0004(80)90268-6
Kennedy C, Burnstock G (1985) Evidence for two types of P2-purinoceptor in longitudinal muscle of the rabbit portal vein. Eur J Pharmacol 111(1):49–56. https://doi.org/10.1016/0014-2999(85)90112-8
Kaczmarek-Hajek K, Lorinczi E, Hausmann R, Nicke A (2012) Molecular and functional properties of P2X receptors--recent progress and persisting challenges. Purinergic Signal 8(3):375–417. https://doi.org/10.1007/s11302-012-9314-7
Surprenant A, Rassendren F, Kawashima E, North RA, Buell G (1996) The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272(5262):735–738. https://doi.org/10.1126/science.272.5262.735
Rodrigues AM, Bergamaschi CT, Fernandes MJ, Paredes-Gamero EJ, Curi MV, Ferreira AT, Araujo SR, Punaro GR, Maciel FR, Nogueira GB, Higa EM (2014) P2x(7) receptor in the kidneys of diabetic rats submitted to aerobic training or to N-acetylcysteine supplementation. PLoS One 9(6):e97452. https://doi.org/10.1371/journal.pone.0097452
Rodrigues AM, Serralha RS, Farias C, Punaro GR, Fernandes MJS, Higa EMS (2018) P2X7 receptor and klotho expressions in diabetic nephropathy progression. Purinergic Signal 14(2):167–176. https://doi.org/10.1007/s11302-018-9602-y
Sugiura H, Yoshida T, Mitobe M, Yoshida S, Shiohira S, Nitta K, Tsuchiya K (2010) Klotho reduces apoptosis in experimental ischaemic acute kidney injury via HSP-70. Nephrol Dial Transplant 25(1):60–68. https://doi.org/10.1093/ndt/gfp451
Shen Y, Yan Y, Lu L, Qian Y, Guan X, Zhang L, Qi Y, Gu L, Ding F (2017) Klotho ameliorates hydrogen peroxide-induced oxidative injury in TCMK-1 cells. Int Urol Nephrol 50(4):787–798. https://doi.org/10.1007/s11255-017-1765-x
Tan SJ, Smith ER, Hewitson TD, Holt SG, Toussaint ND (2014) The importance of klotho in phosphate metabolism and kidney disease. Nephrology (Carlton) 19(8):439–449. https://doi.org/10.1111/nep.12268
Zhou X, Chen K, Lei H, Sun Z (2015) Klotho gene deficiency causes salt-sensitive hypertension via monocyte chemotactic protein-1/CC chemokine receptor 2-mediated inflammation. J Am Soc Nephrol 26(1):121–132. https://doi.org/10.1681/ASN.2013101033
Katsuda Y, Kemmochi Y, Maki M, Sano R, Toriniwa Y, Ishii Y, Miyajima K, Kakimoto K, Ohta T (2014) Effects of unilateral nephrectomy on renal function in male spontaneously diabetic Torii fatty rats: a novel obese type 2 diabetic model. J Diab Res 2014:363126. https://doi.org/10.1155/2014/363126
Lbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411(6836):494–498. https://doi.org/10.1038/35078107
Kumar P, Wu H, McBride JL, Jung KE, Kim MH, Davidson BL, Lee SK, Shankar P, Manjunath N (2007) Transvascular delivery of small interfering RNA to the central nervous system. Nature 448(7149):39–43. https://doi.org/10.1038/nature05901
Hampl V, Walters CL, Archer SL (1996) Determination of nitric oxide by the chemiluminescence reaction with ozone. In: Feelisch M, Stamler JS (eds) Methods in nitric oxide research. John Wiley & Sons, Chichester, pp 310–318
Bernheim F, Bernheim ML, Wilbur KM (1948) The reaction between thiobarbituric acid and the oxidation products of certain lipides. J Biol Chem 174(1):257–264
Shimizu MH, Danilovic A, Andrade L, Volpini RA, Liborio AB, Sanches TR, Seguro AC (2008) N-acetylcysteine protects against renal injury following bilateral ureteral obstruction. Nephrol Dial Transplant 23(10):3067–3073. https://doi.org/10.1093/ndt/gfn237
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Skrzycki M, Majewska M, Podsiad M, Czeczot H (2009) Expression and activity of superoxide dismutase isoenzymes in colorectal cancer. Acta Biochim Pol 56(4):663–670. https://doi.org/10.18388/abp.2009_2500
Apolloni S, Parisi C, Pesaresi MG, Rossi S, Carri MT, Cozzolino M, Volonte C, D'Ambrosi N (2013) The NADPH oxidase pathway is dysregulated by the P2X7 receptor in the SOD1-G93A microglia model of amyotrophic lateral sclerosis. J Immunol 190(10):5187–5195. https://doi.org/10.4049/jimmunol.1203262
Apolloni S, Fabbrizio P, Parisi C, Amadio S, Volonte C (2016) Clemastine confers neuroprotection and induces an anti-inflammatory phenotype in SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Mol Neurobiol 53(1):518–531. https://doi.org/10.1007/s12035-014-9019-8
Freitas HR, Ferraz G, Ferreira GC, Ribeiro-Resende VT, Chiarini LB, do Nascimento JL, Matos Oliveira KR, Pereira Tde L, Ferreira LG, Kubrusly RC, Faria RX, Herculano AM, Reis RA (2016) Glutathione-induced calcium shifts in chick retinal glial cells. PLoS One 11(4):e0153677. https://doi.org/10.1371/journal.pone.0153677 eCollection 2016
Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, Ferrario F, Fogo AB, Haas M, de Heer E, Joh K, Noel LH, Radhakrishnan J, Seshan SV, Bajema IM, Bruijn JA, Renal Pathology S (2010) Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 21(4):556–563. https://doi.org/10.1681/ASN.2010010010
Menzies RI, Booth JWR, Mullins JJ, Bailey MA, Tam FWK, Norman JT, Unwin RJ (2017) Hyperglycemia-induced Renal P2X7 receptor activation enhances diabetes-related injury. EBioMedicine 19:73–83. https://doi.org/10.1016/j.ebiom.2017.04.011
Hou XX, Dong HR, Sun LJ, Yang M, Cheng H, Chen YP (2018) Purinergic 2X7 receptor is involved in the podocyte damage of obesity-related glomerulopathy via activating nucleotide-binding and oligomerization domain-like receptor protein 3 inflammasome. Chin Med J 131(22):2713–2725. https://doi.org/10.4103/0366-6999.245270
Di Bona D, Accardi G, Virruso C, Candore G, Caruso C (2014) Association of klotho polymorphisms with healthy aging: a systematic review and meta-analysis. Rejuvenation Res 17(2):212–216. https://doi.org/10.1089/rej.2013.1523
Li R, Uttarwar L, Gao B, Charbonneau M, Shi Y, Chan JS, Dubois CM, Krepinsky JC (2015) High glucose up-regulates ADAM17 through HIF-1alpha in mesangial cells. J Biol Chem 290(35):21603–21614. https://doi.org/10.1074/jbc.M115.651604
Takenaka T, Kobori H, Inoue T, Miyazaki T, Suzuki H, Nishiyama A, Ishii N, Hayashi M (2020) Klotho supplementation ameliorates blood pressure and renal function in DBA/2-pcy mice, a model of polycystic kidney disease. Am J Physiol Renal Physiol. https://doi.org/10.1152/ajprenal.00299.2019
Yin C, Cheng C, Wang J, Zhang L, Purrunsing Y, Yang G, Zeng M, Huang H, Ren W, Ye Y, Ma H, Xing C, Wang N (2020) The relationship between urinary kidney injury molecule-1 and blood bone metabolism markers in patients with chronic kidney disease. Clin Nephrol 93(2):65–76. https://doi.org/10.5414/CN109763
Lacroix JS, Urena-Torres P (2019) Potential application of fibroblast growth factor 23-klotho axis in chronic kidney disease. Nephrol Ther. https://doi.org/10.1016/j.nephro.2019.05.003
Qian Y, Che L, Yan Y, Lu R, Zhu M, Xue S, Ni Z, Gu L (2019) Urine klotho is a potential early biomarker for acute kidney injury and associated with poor renal outcome after cardiac surgery. BMC Nephrol 20(1):268. https://doi.org/10.1186/s12882-019-1460-5
Cheng MF, Chen LJ, Cheng JT (2010) Decrease of klotho in the kidney of streptozotocin-induced diabetic rats. J Biomed Biotechnol 2010:513853. https://doi.org/10.1155/2010/513853
Asai O, Nakatani K, Tanaka T, Sakan H, Imura A, Yoshimoto S, Samejima K, Yamaguchi Y, Matsui M, Akai Y, Konishi N, Iwano M, Nabeshima Y, Saito Y (2012) Decreased renal alpha-klotho expression in early diabetic nephropathy in humans and mice and its possible role in urinary calcium excretion. Kidney Int 81(6):539–547. https://doi.org/10.1038/ki.2011.423
Hu MC, Shi M, Zhang J, Quinones H, Griffith C, Kuro-o M, Moe OW (2011) Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 22(1):124–136. https://doi.org/10.1681/ASN.2009121311
Zou D, Wu W, He Y, Ma S, Gao J (2018) The role of klotho in chronic kidney disease. BMC Nephrol 19(1):285–212. https://doi.org/10.1186/s12882-018-1094-z
Kacso IM, Bondor CI, Kacso G (2012) Soluble serum klotho in diabetic nephropathy: relationship to VEGF-A. 45(16):1415–1420. https://doi.org/10.1016/j.clinbiochem.2012.07.098
Zhao Y, Banerjee S, Dey N, LeJeune WS, Sarkar PS, Brobey R, Rosenblatt KP, Tilton RG, Choudhary S (2011) Klotho depletion contributes to increased inflammation in kidney of the db/db mouse model of diabetes via RelA (serine)536 phosphorylation. Diabetes 60(7):1907–1916. https://doi.org/10.2337/db10-1262
Saito Y, Yamagishi T, Nakamura T, Ohyama Y, Aizawa H, Suga T, Matsumura Y, Masuda H, Kurabayashi M, Kuro-o M, Nabeshima Y, Nagai R (1998) Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun 248(2):324–329. https://doi.org/10.1006/bbrc.1998.8943
Kim JH, Xie J, Hwang KH, Wu YL, Oliver N, Eom M, Park KS, Barrezueta N, Kong ID, Fracasso RP, Huang CL, Cha SK (2017) Klotho may ameliorate proteinuria by targeting TRPC6 channels in podocytes. J Am Soc Nephrol 28(1):140–151. https://doi.org/10.1681/ASN.2015080888
Acknowledgments
The authors acknowledge Margaret G Mouro for nitric oxide measurements and Professor Sergio R R Araujo for histological analysis.
Funding
This study was supported by Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior (CAPES) and FAPESP (# 2014/26750-9).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
I confirm that all authors read the journal’s policy on disclosure and they have no potential conflict of interest.
Ethical approval
The protocol was approved by the Ethics Committee in Research of Universidade Federal de Sao Paulo under protocol #2056100314.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 258 kb)
Rights and permissions
About this article
Cite this article
Rodrigues, A.M., Serralha, R.S., Lima, D.Y. et al. P2X7 siRNA targeted to the kidneys increases klotho and delays the progression of experimental diabetic nephropathy. Purinergic Signalling 16, 175–185 (2020). https://doi.org/10.1007/s11302-020-09695-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-020-09695-1