Abstract
Background
Lithium remains the first-line treatment for bipolar disorder (BD), but patients respond to it variably. While a myriad of studies have attributed many genes and signaling pathways to lithium responsiveness, a comprehensive study with an integrated conclusion is still lacking.
Objective
We aim to present an integrated mechanism for the therapeutic actions of lithium in BD.
Methods
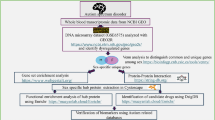
First, a list of lithium responsiveness-associated genes (LRAGs) was collected by searching in the literature. Thereafter, gene set enrichment analysis together with gene–gene interaction network analysis was performed, in order to find the cellular and molecular events related to the LRAGs.
Results
Gene set enrichment analyses showed that the chromosomal regions 3p26, 4p21, 5q34 and 7p13 could be novel associated loci for lithium responsiveness in BD. Also, expression pattern analysis of the LRAGs showed their enrichment in adulthood stages and different cell lineages of brain, blood and immune system. Most of the LRAGs exhibited enriched expression in central parts of human brain, suggesting major contribution of these parts in lithium responsiveness. Beside the prediction of several biological processes and signaling pathways related to lithium responsiveness, an interaction network between these processes was constructed that was found to be regulated by a set of microRNAs. Proteins of the network were mainly classified as transcription factors and kinases, which also highlighted the crucial role of glycogen synthase kinase 3β (GSK3β) in lithium responsiveness.
Conclusions
The predicted cellular and molecular events in this study could be considered as mechanisms and also determinants of lithium responsiveness in BD.





Similar content being viewed by others
References
Sher L. Manic-depressive illness: bipolar disorders and recurrent depression. Am J Psychiatry. 2008;165(4):541–2.
Hou L, Heilbronner U, Degenhardt F, Adli M, Akiyama K, Akula N, et al. Genetic variants associated with response to lithium treatment in bipolar disorder: a genome-wide association study. Lancet. 2016;387(10023):1085–93.
Alda M. Lithium in the treatment of bipolar disorder: pharmacology and pharmacogenetics. Mol Psychiatry. 2015;20(6):661.
Rybakowski JK, Chlopocka-Wozniak M, Suwalska A. The prophylactic effect of long-term lithium administration in bipolar patients entering treatment in the 1970s and 1980s. Bipolar Disord. 1980s;3(2):63–7.
Tighe SK, Mahon PB, Potash JB. Predictors of lithium response in bipolar disorder. Ther Adv Chronic Dis. 2011;2(3):209–26.
Wei Shan G, Makmor-Bakry M, Salihah OM. Long term use of lithium and factors associated with treatment response among patients with bipolar disorder. Psychiatria Danubina. 2016;28(2):146–53.
Kapur V, Nadella RK, Raghuraman BS, Saraf G, Mishra S, Srinivasmurthy N, et al. Clinical factors associated with lithium treatment response in bipolar disorder patients from India. Asian J Psychiatry. 2018;39(38):165–8.
Sportiche S, Geoffroy PA, Brichant-Petitjean C, Gard S, Khan J-P, Azorin J-M, et al. Clinical factors associated with lithium response in bipolar disorders. Aust N Z J Psychiatry. 2017;51(5):524–30.
Aronoff MS, Epstein RS. Factors associated with poor response to lithium carbonate: a clinical study. Am J Psychiatry. 1970;127(4):472–80.
Eugene AR, Masiak J, Eugene B. Predicting lithium treatment response in bipolar patients using gender-specific gene expression biomarkers and machine learning. F1000Research. 2018;7:474–99.
Mertens J, Wang Q-W, Kim Y, Diana XY, Pham S, Yang B, et al. Differential responses to lithium in hyperexcitable neurons from patients with bipolar disorder. Nature. 2015;527(7576):95.
Stern S, Santos R, Marchetto M, Mendes A, Rouleau G, Biesmans S, et al. Neurons derived from patients with bipolar disorder divide into intrinsically different sub-populations of neurons, predicting the patients’ responsiveness to lithium. Mol Psychiatry. 2018;23(6):1453.
Cruceanu C, Alda M, Turecki G. Lithium: a key to the genetics of bipolar disorder. Genome Med. 2009;1(8):79.
Manchia M, Adli M, Akula N, Ardau R, Aubry J-M, Backlund L, et al. Assessment of response to lithium maintenance treatment in bipolar disorder: a Consortium on Lithium Genetics (ConLiGen) report. PLoS One. 2013;8(6):e65636.
Schulze TG, Alda M, Adli M, Akula N, Ardau R, Bui ET, et al. The International Consortium on Lithium Genetics (ConLiGen): an initiative by the NIMH and IGSLI to study the genetic basis of response to lithium treatment. Neuropsychobiology. 2010;62(1):72–8.
Berridge MJ. Inositol 1, 4, 5-trisphosphate-induced calcium mobilization is localized in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1989;238(1292):235–43.
Berridge MJ, Downes CP, Hanley MR. Neural and developmental actions of lithium: a unifying hypothesis. Cell. 1989;59(3):411–9.
Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci. 1996;93(16):8455–9.
Benedetti F, Bernasconi A, Lorenzi C, Pontiggia A, Serretti A, Colombo C, et al. A single nucleotide polymorphism in glycogen synthase kinase 3-β promoter gene influences onset of illness in patients affected by bipolar disorder. Neurosci Lett. 2004;355(1–2):37–40.
Benedetti F, Serretti A, Pontiggia A, Bernasconi A, Lorenzi C, Colombo C, et al. Long-term response to lithium salts in bipolar illness is influenced by the glycogen synthase kinase 3-β-50 T/C SNP. Neurosci Lett. 2005;376(1):51–5.
Song J, Bergen S, Di Florio A, Karlsson R, Charney A, Ruderfer D, et al. Genome-wide association study identifies SESTD1 as a novel risk gene for lithium-responsive bipolar disorder. Mol Psychiatry. 2016;21(9):1290.
McCarthy MJ, Leckband SG, Kelsoe JR. Pharmacogenetics of lithium response in bipolar disorder. Pharmacogenomics. 2010;11(10):1439–65.
Papiol S, Schulze TG, Alda M. Genetics of lithium response in bipolar disorder. Pharmacopsychiatry. 2018;51(5):206–11.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9.
Buyske S, Bates ME, Gharani N, Matise TC, Tischfield JA, Manowitz P. Cognitive traits link to human chromosomal regions. Behav Genet. 2006;36(1):65.
Franchini LF, Pollard KS. Genomic approaches to studying human-specific developmental traits. Development. 2015;142(18):3100–12.
Li H, Chen H, Liu F, Ren C, Wang S, Bo X, et al. Functional annotation of HOT regions in the human genome: implications for human disease and cancer. Sci Rep. 2015;5:11633.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci. 2005;102(43):15545–50.
Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013;14(1):128.
Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44(W1):W90–W9797.
Palasca O, Santos A, Stolte C, Gorodkin J, Jensen LJ. TISSUES 2.0: an integrative web resource on mammalian tissue expression. Database. 2018;2018:1–12.
Sunkin SM, Ng L, Lau C, Dolbeare T, Gilbert TL, Thompson CL, et al. Allen Brain Atlas: an integrated spatio-temporal portal for exploring the central nervous system. Nucleic Acids Res. 2012;41(D1):D996–D1008.
Jones AR, Overly CC, Sunkin SM. The Allen brain atlas: 5 years and beyond. Nat Rev Neurosci. 2009;10(11):821.
Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2008;4(1):44.
Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2008;37(1):1–13.
Mi H, Muruganujan A, Thomas PD. PANTHER in 2013: modeling the evolution of gene function, and other gene attributes, in the context of phylogenetic trees. Nucleic Acids Res. 2012;41(D1):D377–D386386.
Thomas PD, Campbell MJ, Kejariwal A, Mi H, Karlak B, Daverman R, et al. PANTHER: a library of protein families and subfamilies indexed by function. Genome Res. 2003;13(9):2129–41.
Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, et al. The STRING database in 2017: quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res. 2016;45(1):362–8.
Kerner B, Brugman DL, Freimer NB. Evidence of linkage to psychosis on chromosome 5q33-34 in pedigrees ascertained for bipolar disorder. Am J Med Genet Part B Neuropsychiatr Genet. 2007;144(1):74–8.
Herzberg I, Jasinska A, García J, Jawaheer D, Service S, Kremeyer B, et al. Convergent linkage evidence from two Latin-American population isolates supports the presence of a susceptibility locus for bipolar disorder in 5q31–34. Hum Mol Genet. 2006;15(21):3146–53.
Hamshere ML, Schulze TG, Schumacher J, Corvin A, Owen MJ, Jamra RA, et al. Mood-incongruent psychosis in bipolar disorder: conditional linkage analysis shows genome-wide suggestive linkage at 1q3.23, 7p13 and 20q13.31. Bipolar Disord. 2009;11(6):610–20.
Liu J, Juo S, Dewan A, Grunn A, Tong X, Brito M, et al. Evidence for a putative bipolar disorder locus on 2p13–16 and other potential loci on 4q31, 7q34, 8q13, 9q31, 10q21–24, 13q32, 14q21 and 17q11–12. Mol Psychiatry. 2003;8(3):333.
Pergadia ML, Glowinski AL, Wray NR, Agrawal A, Saccone SF, Loukola A, et al. A 3p26-3p25 genetic linkage finding for DSM-IV major depression in heavy smoking families. Am J Psychiatry. 2011;168(8):848–52.
Lewis CM, Levinson DF, Wise LH, DeLisi LE, Straub RE, Hovatta I, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. Am J Hum Genet. 2003;73(1):34–48.
Kerner B, Lambert CG, Muthen BO. Genome-wide association study in bipolar patients stratified by co-morbidity. PLoS One. 2011;6(12):e28477.
Chen C-H, Lee C-S, Lee M-TM, Ouyang W-C, Chen C-C, Chong M-Y, et al. Variant GADL1 and response to lithium therapy in bipolar I disorder. N Engl J Med. 2014;370(2):119–28.
Zanni G, Michno W, Di Martino E, Tjärnlund-Wolf A, Pettersson J, Mason CE, et al. Lithium accumulates in neurogenic brain regions as revealed by high resolution ion imaging. Sci Rep. 2017;7:40726.
Thellier M, Wissocq J, Heurteaux C. Quantitative microlocation of lithium in the brain by a (n, α) nuclear reaction. Nature. 1980;283(5744):299.
Thellier M, Heurteaux C, Wissocq J-C. Quantitative study of the distribution of lithium in the mouse brain for various doses of lithium given to the animal. Brain Res. 1980;199(1):175–96.
Smith FE, Thelwall PE, Necus J, Flowers CJ, Blamire AM, Cousins DA. 3D 7 Li magnetic resonance imaging of brain lithium distribution in bipolar disorder. Mol Psychiatry. 2018;23(11):2184.
Maddu N, Raghavendra PB. Review of lithium effects on immune cells. Immunopharmacol Immunotoxicol. 2015;37(2):111–25.
Takaesu Y. Circadian rhythm in bipolar disorder: a review of the literature. Psychiatry Clin Neurosci. 2018;72(9):673–82.
Melo MC, Abreu RL, Neto VBL, de Bruin PF, de Bruin VM. Chronotype and circadian rhythm in bipolar disorder: a systematic review. Sleep Med Rev. 2017;34:46–58.
Gallicchio VS. Effects of lithium on cell growth. Lithium and cell physiology. Berlin: Springer; 1990. p. 121–124.
Smits VA, Essers MA, Loomans DS, Klompmaker R, Rijksen G, Medema RH. Inhibition of cell proliferation by lithium is associated with interference in cdc2 activation. FEBS Lett. 1999;457(1):23–7.
de Groot T, Alsady M, Jaklofsky M, Otte-Höller I, Baumgarten R, Giles RH, et al. Lithium causes G2 arrest of renal principal cells. J Am Soc Nephrol. 2014;25(3):501–10.
Zhang W, Jüllig M, Connolly A, Stott N. Early gene response in lithium chloride induced apoptosis. Apoptosis. 2005;10(1):75–90.
Pietruczuk K, Jóźwik A, Ruckemann-Dziurdzińska K, Bryl E, Witkowski J. Cytoprotective effect of lithium against spontaneous and induced apoptosis of lymphoid cell line MOLT-4. Folia Histochem Cytobiol. 2009;47(4):639–46.
Uribe E, Wix R. Neuronal migration, apoptosis and bipolar disorder. Revista de Psiquiatría y Salud Mental (English Edition). 2012;5(2):127–33.
Fries GR, Vasconcelos-Moreno MP, Gubert C, Dos Santos BTMQ, Da Rosa ALST, Eisele B, et al. Early apoptosis in peripheral blood mononuclear cells from patients with bipolar disorder. J Affect Disord. 2014;152:474–7.
Coyle JT, Duman RS. Finding the intracellular signaling pathways affected by mood disorder treatments. Neuron. 2003;38(2):157–60.
Brown KM, Tracy DK. Lithium: the pharmacodynamic actions of the amazing ion. Ther Adv Psychopharmacol. 2013;3(3):163–76.
Feng H-L, Leng Y, Ma C-H, Zhang J, Ren M, Chuang D-M. Combined lithium and valproate treatment delays disease onset, reduces neurological deficits and prolongs survival in an amyotrophic lateral sclerosis mouse model. Neuroscience. 2008;155(3):567–72.
Spiliotaki M, Salpeas V, Malitas P, Alevizos V, Moutsatsou P. Altered glucocorticoid receptor signaling cascade in lymphocytes of bipolar disorder patients. Psychoneuroendocrinology. 2006;31(6):748–60.
Watson S, Thompson JM, Ritchie JC, Nicol Ferrier I, Young AH. Neuropsychological impairment in bipolar disorder: the relationship with glucocorticoid receptor function. Bipolar Disord. 2006;8(1):85–90.
Spijker A, Van Rossum E. Glucocorticoid sensitivity in mood disorders. Neuroendocrinology. 2012;95(3):179–86.
Zhou R, Gray NA, Yuan P, Li X, Chen J, Chen G, et al. The anti-apoptotic, glucocorticoid receptor cochaperone protein BAG-1 is a long-term target for the actions of mood stabilizers. J Neurosci. 2005;25(18):4493–502.
Miyazaki I, Nagamachi T, Shinomiya K, Matsunaga H, Sendo T, Kawasaki H, et al. Effects of imipramine and lithium on the suppression of cell proliferation in the dentate gyrus of the hippocampus in adrenocorticotropic hormone-treated rats. Acta Med Okayama. 2010;64(4):219–23.
Sigitova E, Fišar Z, Hroudová J, Cikánková T, Raboch J. Biological hypotheses and biomarkers of bipolar disorder. Psychiatry Clin Neurosci. 2017;71(2):77–103.
Hannestad JO, Cosgrove KP, DellaGioia NF, Perkins E, Bois F, Bhagwagar Z, et al. Changes in the cholinergic system between bipolar depression and euthymia as measured with [123I] 5IA single photon emission computed tomography. Biol Psychiatry. 2013;74(10):768–76.
Ashok AH, Marques TR, Jauhar S, Nour MM, Goodwin G, Young AH, et al. The dopamine hypothesis of bipolar affective disorder: the state of the art and implications for treatment. Mol Psychiatry. 2017;22(5):666.
Can A, Frost DO, Cachope R, Cheer JF, Gould TD. Chronic lithium treatment rectifies maladaptive dopamine release in the nucleus accumbens. J Neurochem. 2016;139(4):576–85.
Vizi E, Illes P, Ronai A, Knoll J. The effect of lithium on acetylcholine release and synthesis. Neuropharmacology. 1972;11(4):521–30.
Dehpour A, Farsam H, Azizabadi-Farahani M. Inhibition of the morphine withdrawal syndrome and the development of physical dependence by lithium in mice. Neuropharmacology. 1995;34(1):115–21.
Budde M, Degner D, Brockmöller J, Schulze T. Pharmacogenomic aspects of bipolar disorder: an update. Eur Neuropsychopharmacol. 2017;27(6):599–609.
Liu S, Zhang F, Wang X, Shugart YY, Zhao Y, Li X, et al. Diagnostic value of blood-derived microRNAs for schizophrenia: results of a meta-analysis and validation. Sci Rep. 2017;7(1):15328.
Reinbold CS, Forstner AJ, Hecker J, Fullerton JM, Hoffmann P, Hou L, et al. Analysis of the influence of microRNAs in lithium response in bipolar disorder. Front Psychiatry. 2018;9:207.
Chen H, Wang N, Burmeister M, McInnis MG. MicroRNA expression changes in lymphoblastoid cell lines in response to lithium treatment. Int J Neuropsychopharmacol. 2009;12(7):975–81.
Hunsberger J, Chibane F, Elkahloun A, Henderson R, Singh R, Lawson J, et al. Novel integrative genomic tool for interrogating lithium response in bipolar disorder. Transl Psychiatry. 2015;5(2):e504.
Chao Y-L, Chen C-H. An introduction to microRNAs and their dysregulation in psychiatric disorders. Tzu Chi Medical Journal. 2013;25(1):1–7.
Kim AH, Reimers M, Maher B, Williamson V, McMichael O, McClay JL, et al. MicroRNA expression profiling in the prefrontal cortex of individuals affected with schizophrenia and bipolar disorders. Schizophr Res. 2010;124(1–3):183–91.
Lawrie CH. MicroRNA expression in lymphoid malignancies: new hope for diagnosis and therapy? J Cell Mol Med. 2008;12(5a):1432–44.
Biswas S, Haleyurgirisetty M, Lee S, Hewlett I, Devadas K. Development and validation of plasma miRNA biomarker signature panel for the detection of early HIV-1 infection. EBioMedicine. 2019;43:307–16.
Zhou R, Yuan P, Wang Y, Hunsberger JG, Elkahloun A, Wei Y, et al. Evidence for selective microRNAs and their effectors as common long-term targets for the actions of mood stabilizers. Neuropsychopharmacology. 2009;34(6):1395.
Malhi GS, Tanious M, Das P, Berk M. The science and practice of lithium therapy. Aust N Z J Psychiatry. 2012;46(3):192–21111.
Malhi GS, Outhred T. Therapeutic mechanisms of lithium in bipolar disorder: recent advances and current understanding. CNS Drugs. 2016;30(10):931–49.
Acknowledgements
The authors thank the Allen Institute for Brain Science (551 North 34th Street, Seattle, Washington 98103, USA) and the Broad Institute (415 Main Street, Cambridge, Massachusetts 02142, USA) for their valuable data, which are publicly available for analyses. The authors also thank Bahman Razi (PhD candidate in Hematology, Department of Hematology and Blood Transfusion, Faculty of Medicine, Tarbiat Modares University, Tehran, Iran) for his kind scientific comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding has been received for conducting this study and/or preparation of this manuscript.
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
40263_2020_723_MOESM1_ESM.xlsx
Supplemental file S1. The list of the lithium responsiveness-associated genes (LRAGs), collected from previously published articles. These genes are listed by their official symbols and also by their full names (XLSX 17 kb)
40263_2020_723_MOESM2_ESM.xlsx
Supplemental file S2. A subset of LRAGs that were specifically enriched in four signaling pathways. These genes were used as the input data of PANTHER analysis of protein classes. LRAGs lithium responsiveness-associated genes, PANTHER Protein ANalysis THrough Evolutionary Relationships (XLSX 10 kb)
40263_2020_723_MOESM3_ESM.pdf
Supplemental figure S1. Circadian rhythm signaling pathway (KEGG number: map04710). The first-ranked enriched signaling pathway for the LRAGs was circadian rhythm pathway. It consists of cell-autonomous transcription-translation feedback loops that drives rhythmic expression patterns of core clock components. The first negative feedback loop is a rhythmic transcription of period (Per) genes and chryptochrome (Cry) genes. Per and Cry proteins form a heterodimer, which acts on the Clock/Bmal1 heterodimer to repress its own transcription. Per and Cry proteins are phosphorylated by casein kinase epsilon (CkIε), which leads to degradation and restarting of the cycle. The second loop is a positive feedback loop, driven by the Clock/Bmal1 heterodimer, which initiates transcription of target genes containing E-box cis-regulatory enhancer sequences. The components with the red stars denote LRAGs. KEGG Kyoto Encyclopedia of Genes and Genomes, LRAGs lithium responsiveness-associated genes (PDF 1056 kb)
40263_2020_723_MOESM4_ESM.pdf
Supplemental figure S2. Synaptic signaling pathways and their LRAGs. (a) Signaling by a dopaminergic synapse (KEGG number: map04728). (b) Signaling by a cholinergic synapse (KEGG number: map04725). In both pathways, signaling events are shared between neuronal (bottom left and bottom right cells) and glial cells (top). A significant number of LRAGs were enriched for these pathways (denoted as red asterisks). KEGG Kyoto Encyclopedia of Genes and Genomes, LRAGs lithium responsiveness-associated genes (PDF 1922 kb)
40263_2020_723_MOESM5_ESM.pdf
Supplemental figure S3. The signaling pathway of morphine addiction (KEGG number: map05032). The interactions between components of this pathway conditions are illustrated in five different conditions: (a) in the absence of morphine (control). (b) Acute use of morphine. (c) Chronic use of morphine. (d) Withdrawal from chronic use of morphine. (e) Increased adenosine tone, mediated by morphine withdrawal. Red asterisks denote the LRAGs of the pathway. KEGG Kyoto Encyclopedia of Genes and Genomes, LRAGs lithium responsiveness-associated genes (PDF 2827 kb)
Rights and permissions
About this article
Cite this article
Najafi, H., Totonchi, M. & Sadeghizadeh, M. Predicted Cellular and Molecular Actions of Lithium in the Treatment of Bipolar Disorder: An In Silico Study. CNS Drugs 34, 521–533 (2020). https://doi.org/10.1007/s40263-020-00723-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-020-00723-7