Abstract
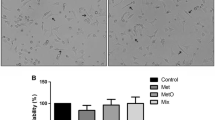
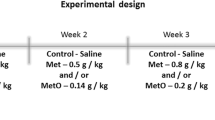
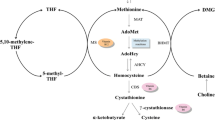
Hypermethioninemia is a disorder characterized by high plasma levels of methionine (Met) and its metabolites such as methionine sulfoxide (MetO). Studies have reported associated inflammatory complications, but the mechanisms involved in the pathophysiology of hypermethioninemia are still uncertain. The present study aims to evaluate the effect of chronic administration of Met and/or MetO on phenotypic characteristics of macrophages, in addition to oxidative stress, purinergic system, and inflammatory mediators in macrophages. In this study, Swiss male mice were subcutaneously injected with Met and MetO at concentrations of 0.35–1.2 g/kg body weight and 0.09–0.3 g/kg body weight, respectively, from the 10th–38th day post-birth, while the control group was treated with saline solution. The results revealed that Met and/or MetO induce an M1/classical activation phenotype associated with increased levels of tumor necrosis factor alpha and nitrite, and reduced arginase activity. It was also found that Met and/or MetO alter the activity of antioxidant enzymes superoxide dismutase, catalase, and glutathione peroxidase, as well as the levels of thiol and reactive oxygen species in macrophages. The chronic administration of Met and/or MetO also promotes alteration in the hydrolysis of ATP and ADP, as indicated by the increased activity of ectonucleotidases. These results demonstrate that chronic administration of Met and/or MetO promotes activated pro-inflammatory profile by inducing M1/classical macrophage polarization. Thus, the changes in redox status and purinergic system upon chronic Met and/or MetO exposure may contribute towards better understanding of the alterations consistent with hypermethioninemic patients.




Similar content being viewed by others
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Aksenov MY, Markesbery WR (2001) Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer's disease. Neurosci Lett 302:141–145
Allen J, Power B, Abedin A, Purcell O, Knerr I, Monavari A (2019) Plasma methionine concentrations and incidence of hypermethioninemic encephalopathy during infancy in a large cohort of 36 patients with classical homocystinuria in the Republic of Ireland. JIMD Rep 47:41–46
Antonioli L, Csóka B, Fornai M, Colucci R, Kokai E, Blandizzi C, Haskó G (2014) Adenosine and inflammation: what's new on the horizon? Drug Discov Today 19:1051–1068
Araki A, Sako Y (1987) Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr B Biomed Sci Appl 422:43–52
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Catorce MN, Gevorkian G (2016) LPS-induced Murine Neuroinflammation model: main features and suitability for pre-clinical assessment of nutraceuticals. Curr Neuropharmacol 14:155–164
Chan KM, Delfert D, Junger KD (1986) A direct colorimetric assay for Ca2+-stimulated ATPase activity. Anal Biochem 157:375–380
Corraliza IM, Campo ML, Soler G, Modolell M (1994) Determination of arginase activity in macrophages: a micromethod. J Immunol Methods 174:231–235
Costa MZ, Da Silva TM, Flores NP, Schmitz F, Scherer EB, Viau CM, Saffi J, Barschak AG, Wyse AT, Spanevello RM, Stefanello FM (2013) Methionine and methionine sulfoxide alter parameters of oxidative stress in the liver of young rats: in vitro and in vivo studies. Mol Cell Biochem 384:21–28
Dosch M, Gerber J, Jebbawi F, Beldi G (2018) Mechanisms of ATP release by inflammatory cells. Int J Mol Sci 19:1222
Dosch M, Zindel J, Jebbawi F, Melin N, Sanchez-Taltavull D, Stroka D, Candinas D, Beldi G (2019) Connexin-43-dependent ATP release mediates macrophage activation during sepsis. Elife 8:e42670
Dos Santos LM, Da Silva TM, Azambuja JH, Ramos PT, Oliveira PS, Da Silveira EF, Pedra NS, Galdino K, Do Couto CAT, Soares MSP, Tavares RG, Spanevello RM, Stefanello FM, Braganhol E (2017) Methionine and methionine sulfoxide treatment induces M1/classical macrophage polarization and modulates oxidative stress and purinergic signaling parameters. Mol Cell Biochem 424:69–78
Gaull GE, Tallan HH (1974) Methionine adenosyltransferase deficiency: new enzymatic defect associated with hypermethioninemia. Science 186:59–60
Halliwell B (2011) Free radicals and antioxidants–quo vadis? Trends Pharmacol Sci 32:125–130
Idzko M, Ferrari D, Eltzschig HK (2014) Nucleotide signalling during inflammation. Nature 509:310
Joseph MH, Marsden CA (1986) Amino acids and small peptides. HPLC of small peptides. IRL Press, Oxford 13–27.
Junger WG (2011) Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol 11:201
Li CQ, Barshop BA, Feigenbaum A, Khanna PC (2018a) Brain magnetic resonance imaging findings in poorly controlled homocystinuria. J Radiol Case Rep 12:1–8
Li Z, Wang L, Liu H, Muyayalo KP, Huang X, Mor G, Liao A (2018b) Galectin-9 alleviates LPS-induced preeclampsia-like impairment in rats via switching decidual macrophage polarization to M2 subtype. Front Immunol 9:3142
Lugrin J, Rosenblatt-Velin N, Parapanov R, Liaudet L (2014) The role of oxidative stress during inflammatory processes. J Biol Chem 395:203–230
Luo S, Levine RL (2009) Methionine in proteins defends against oxidative stress. FASEB J 23:464–472
Maclean KN, Jiang H, Greiner LS, Allen RH, Stabler SP (2012) Long-term betaine therapy in a murine model of cystathionine beta-synthase deficient homocystinuria: decreased efficacy over time reveals a significant threshold effect between elevated homocysteine and thrombotic risk. Mol Genet Metab 105:395–403
Martins E, Marcao A, Bandeira A, Fonseca H, Nogueira C, Vilarinho L (2012) Methionine adenosyltransferase I/III deficiency in Portugal: high frequency of a dominantly inherited form in a small area of Douro High Lands. JIMD Rep 6:107–112
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8:958
Mudd SH (2011) Hypermethioninemias of genetic and non-genetic origin: a review. Am J Med Genet Part C (Seminars in Medical Genetics) 157:3–32
Mudd SH, Jenden DJ, Capdevila A, Roch M, Levy HL, Wagner C (2000) Isolated hypermethioninemia: measurements of S-adenosylmethionine and choline. Metabolism 49:1542–1547
Mudd SH, Levy HL, Skovby F. Disorders of transsulfuration. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill, 2001, vol. 2, pp 1279–1327.
Mudd SH, Tangerman A, Stabler SP, Allen RH, Wagner C, Zeisel SH, Levy HL (2003) Maternal methionine adenosyltransferase I/III deficiency: reproductive outcomes in a woman with four pregnancies. JIMD 26:443–458
Nashabat M, Al-Khenaizan S, Alfadhel M (2018) Methionine adenosyltransferase I/III deficiency: beyond the central nervous system manifestations. Ther Clin Risk Manag 14:225
Robson SC, Sévigny J, Zimmermann H (2006) The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signalling 2:409
Schweinberger BM, Wyse AT (2016) Mechanistic basis of hypermethioninemia Amino acids 48:2479–2489
Schweinberger BM, Schwieder L, Scherer E, Sitta A, Vargas CR, Wyse AT (2014) Development of an animal model for gestational hypermethioninemia in rat and its effect on brain Na+, K+-ATPase/Mg 2+-ATPase activity and oxidative status of the offspring. Metab Brain Dis 29:153–160
Schweinberger BM, Turcatel E, Rodrigues AF, Wyse AT (2015) Gestational hypermethioninaemia alters oxidative/nitrative status in skeletal muscle and biomarkers of muscular injury and inflammation in serum of rat offspring. Int J Exp Pathol 96:277–284
Schweinberger BM, Rodrigues AF, Turcatel E, Pierozan P, Pettenuzzo LF, Grings M, Scaini G, Parisi MM, Leipnitz G, Streck EL, Barbé-Tuana FM, Wyse ATS (2018) Maternal hypermethioninemia affects neurons number, neurotrophins levels, energy metabolism, and Na+, K+-ATPase expression/content in brain of rat offspring. Mol Neurobiol 55:980–988
Soares MSP, Oliveira PS, Debom GN, Da Silveira BM, Polachini CR, Baldissarelli J, Morsch VM, Schetinger MRC, Tavares RG, Stefanello FM, Spanevello RM (2017a) Chronic administration of methionine and/or methionine sulfoxide alters oxidative stress parameters and ALA-D activity in liver and kidney of young rats. Amino Acids 49:129–138
Soares MSP, Viau CM, Saffi J, Costa MZ, Da Silva TM, Oliveira PS, Azambuja JH, Barschak AG, Braganhol E, Wyse ATS, Stefanello FM, Spanevello RM (2017b) Acute administration of methionine and/or methionine sulfoxide impairs redox status and induces apoptosis in rat cerebral cortex. Metab Brain Dis 32:1693–1703
Soares MSP, Costa MZ, da Silva TM, Gazal M, do Couto CAT, Debom GN, Rodrigues R, Azambuja JH, Casali EA, Moritz CEJ, Duarte MF, Braganhol E, Stefanello FM, Spanevello RM (2018) Methionine and/or methionine sulfoxide alter ectoenzymes activities in lymphocytes and inflammatory parameters in serum from young rats: acute and chronic effects. Cell Biochem Biophys 76:243–253
Soares MSP, Mattos BS, Ávila AA, Spohr L, Pedra NS, Teixeira FC, Bona NP, Oliveira PS, Stefanello FM, Spanevello RM (2019) High levels of methionine and methionine sulfoxide: impact on adenine nucleotide hydrolysis and redox status in platelets and serum of young rats. J Cell Biochem 120:2289–2303
Stefanello FM, Chiarani F, Kurek AG, Wannmacher CM, Wajner M, Wyse AT (2005) Methionine alters Na+, K+-ATPase activity, lipid peroxidation and nonenzymatic antioxidant defenses in rat hippocampus. Int J Dev Neurosci 23:651–656
Stefanello FM, Matté C, Scherer EB, Wannmacher CM, Wajner M, Wyse AT (2007a) Chemically induced model of hypermethioninemia in rats. J Neurosci Methods 160:1–4
Stefanello FM, Scherer EB, Kurek AG, Mattos CB, Wyse AT (2007b) Effect of hypermethioninemia on some parameters of oxidative stress and on Na+, K+-ATPase activity in hippocampus of rats. Metab Brain Dis 22:172–182
Stefanello FM, Matté C, Pederzolli CD, Kolling J, Mescka CP, Lamers ML, Assis AM, Perry ML, Santos MF, Dutra-Filho CS, Wyse AT (2009) Hypermethioninemia provokes oxidative damage and histological changes in liver of rats. Biochimie 91:961–968
Stefanello FM, Ferreira AG, Pereira TC, da Cunha MJ, Bonan CD, Bogo MR, Wyse AT (2011) Acute and chronic hypermethioninemia alter Na+, K+-ATPase activity in rat hippocampus: prevention by antioxidants. Int J Dev Neurosci 29:483–488
Stuehr DJ, Nathan CF (1989) Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med 169:1543–1555
Suzuki S, Kodera Y, Saito T, Fujimoto K, Momozono A, Hayashi A, Kamata Y, Shichiri M (2016) Methionine sulfoxides in serum proteins as potential clinical biomarkers of oxidative stress. Sci Rep 6:38299
Varol C, Mildner A, Jung S (2015) Macrophages: development and tissue specialization. Annu Rev Immunol 33:643–675
Yoon SY, Hong GH, Kwon HS, Park S, Park SY, Shin B, Kim TB, Moon HB, Cho YS (2016) S-Adenosylmethionine reduces airway inflammation and fibrosis in a murine model of chronic severe asthma via suppression of oxidative stress. Exp Mol Med 48:236
Acknowledgements
This research was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq—Universal Processo 454262/2014-0) and Fundação de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS). This study was financed in part by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior- Brasil (CAPES)—Finance code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical approval
All animal procedures were approved by the Ethics Committee of Animal Experimentation from the Federal University of Pelotas (protocol under project number: CEEA 9221-2013). The use of the animals was in accordance with the Brazilian Guidelines for the Care and Use of Animals in Scientific Research Activities (DBCA), National Council of Control of Animal Experimentation (CONCEA) and with the NIH Guide for Care and Use of Laboratory Animals.
Additional information
Handling Editor: J. Marshal.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Franceschi, T.S., Soares, M.S.P., Pedra, N.S. et al. Characterization of macrophage phenotype, redox, and purinergic response upon chronic treatment with methionine and methionine sulfoxide in mice. Amino Acids 52, 629–638 (2020). https://doi.org/10.1007/s00726-020-02841-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-020-02841-4