Abstract
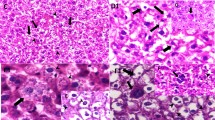
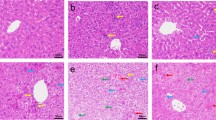
Cadmium is primarily utilized in the construction of particles known as quantum dots. Hepatotoxicity caused by microparticles of cadmium is very well known; however, toxicity of nanoparticles of cadmium is not well understood. The present study describes the toxicity of cadmium sulfide nanoparticles (CdSNPs) in the liver of rat. Adult Wistar rats were administered CdSNPs (10 mg/kg) on alternate days for 45 days. Serum enzymes (ALT, AST, ALP), biomarkers of lipid peroxidation (MDA, H2O2, and NO), and metallothionein concentration were determined. Histopathological and TEM observations were also made to record morphological changes. CdSNPs (10 mg/kg) induced significant changes in the structure and function of liver. Values of serum enzymes and reactive species increased significantly in rats treated with CdSNPs in comparison to CdS-treated rats. Histopathological observations showed extensive parenchymal degeneration. Ultrastructural studies exhibited proliferation of endoplasmic reticulum, microsomes, and lysosomes. It is concluded that NP-membrane interaction leads to the generation of reactive species that alter membrane integrity and induce oxidative stress. These events may activate cell death pathways in hepatocytes.




Similar content being viewed by others
References
Mamalis AG (2007) Recent advances in nanotechnology. J Mater Process Technol 181:52–58
Oberdorster G, Oberdorster E, Oberdorster J (2005) Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 113:823–839. https://doi.org/10.1289/ehp.7339
Peter HH, Irene BH, Oleg VS (2004) Nanoparticles-known and unknown health risks. J.Nanobiotechnology. 2:12. https://doi.org/10.1186/1477-3155-2-12
Davoren M, Herzog E, Casey A, Benjamin C, Gordon C, Hugh JB, Fiona ML (2007) In vitro toxicity evaluation of single walled carbon nanotubes on human A549 lung cells. Toxicol in Vitro 21:438–448. https://doi.org/10.1016/j.tiv.2006.10.007
Hulderman LZ, Salmen T, Chapmen R, Stephen R, Shih-Houng SL, Shvedova Y, Luster MIA, Simeonuva PP (2007) Cardiovascular effects of pulmonary exposure to single wall carbon nanotubes. Environ Health Perspect 115(3):377–382
Stern ST, McNeil ES (2008) Nanotechnology safety concerns revisited. Toxicol Sci 101:4–21. https://doi.org/10.1093/toxsci/kfm169
De Jong WH, Hagens WI, Krystek P, Burger MC, Sips AJ, Geertsma RE (2008) Particle size dependent organ distribution of gold nanoparticles after intravenous administration. Biomater. 29:1912–1919. https://doi.org/10.1016/j.biomaterials
Jeffrey WC, Zeldin DC, Bonner JC, Nestmann RE (2008) Pulmonary applications and toxicity of engineered nanoparticles. Am J Physiol-Lung C 295:1–55
Sintov AC, Velasco-Agerirre C, Gallardo-Toledo E, Araya E, Kogan MJ (2016) Metal nanoparticles as targeted carriers circumventing the blood–brain barrier. Int Rev Neurobiol 130:199–227. https://doi.org/10.1016/bs.irn.2016.06.007
Smith AM, Duan H, Mobs AM, Nile S (2008) Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv Drug Deliv Rev 60:1226–1240. https://doi.org/10.1016/j.addr.2008.03.015
Juzenas P, Chen W, Sun YP, Codho MAN, Genralov R, Genraloves N, Christensen IL (2008) Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer. Adv Drug Deliv Rev 60:1600–1614. https://doi.org/10.1016/j.addr.2008.08.004
Alivistas P (2004) The use of nanocrystals in biological detection. Nat.Biotechnol. 22:47–52. https://doi.org/10.1038/nbt927
Hardman RA (2006) Toxicologic review of quantum dots: toxicity depends upon physic-chemical and environmental factors. Environ.healthPerspect 114:165–172. https://doi.org/10.1289/ehp.8284
Paunovic J, Vucevic D, Radosavljevic T, Pantic S, Nikolovski D, Dugalic S, Pentavi I (2017) Effect of metallic nanoparticles on physiological liver functions. Rev Adv Mater Sci 49:123–128
Valko M, Morris H, Cronin MT (2005) Metals toxicity and oxidative stress. Curr Med Chem 12:1161–1208. https://doi.org/10.2174/0929867053764635
Thevenod F, Lee WK (2013) Cadmium and cellular signaling cascades: interaction between cell death and survival pathways. Arch.Toxicol. 87:1743–1786. https://doi.org/10.1007/s00204-013-1110-9
Thevenod F (2009) Cadmium and cellular signaling cascades: to be or not to be? Toxicol Appl Pharmacol 238:221–239. https://doi.org/10.1016/j.taap.2009.01.013
Rana K, Verma Y, Rani V, Rana SVS (2018) Renal toxicity of nanoparticles of cadmium sulphide in rat. Chemosphere. 193:142–150. https://doi.org/10.1016/j.chemosphere.2017.11.011
Reitman S, Frankel AS (1957) Colorimetric method for determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:56–63. https://doi.org/10.1093/ajcp/28.1.56
King EJ, Armstrong AR (1934) A convenient method for determining serum and bile phosphatase activity. Can Med Assoc J 31:376–381
Onosaka S, Cherian MG (1982) The induced synthesis of metallothionein in various tissues of rats in response to metals. II. Influence of zinc status and specific effect on pancreatic metallothionein. Toxicology 23(1):11–20. https://doi.org/10.1016/0300-483x(82)90037-3
Rana SVS, Kumar A (2000) Metallothionein induced by cadmium or zinc inhibits lipid peroxidation in rats exposed to dimethylnitrosamine. Arh Hig Rada Toksikol 51(3):279–286
Jordan RA, Schenkman JB (1982) Relationship between malondialdehyde production and arachidonate consumption during NADPH-supported microsomal lipid peroxidation. Biochem Pharmacol 31(7):1393–1400. https://doi.org/10.1016/0006-2952(82)90034-X
O.H. Lowry, N.J. Rosebrough, A. L. Farr, RJ Randall, Protein measurement with the Folin phenol reagent, J Biol Chem 193 (1) (1951) 265–275
Thurman RG, Ley HG, Scholz R (1972) Hepatic microsomal ethanol oxidation, hydrogen peroxide formation and the role of catalase. Eur J Biochem 25:420–430. https://doi.org/10.1111/j.1432-1033.1972.tb01711.x
Cortas NK, Wakid NW (1990) Determination of inorganic nitrate in serum and urine by a kinetic cadmium-reduction method. Clin Chem 36:1440–1443
Ellman GL (1959) Tissue sulphydryl groups. Arch Biochem Biophys 82:70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Murata I, Hirono T, Saeki Y, Nakagawa S (1970) Cadmium enteropathy, renal osteomalacia (“ItaiItai” disease in Japan). Bull Soc Int Chir 29(1):34–42
Kobayashi J, Nakahara H, Hasegawa T (1971) Accumulation of cadmium in organs of mice fed on cadmium-polluted rice. Jap J Hyg 26:401–407. https://doi.org/10.1265/jjh.26.401
Liu J, Qu W, Kadiiska MB (2009) Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol Appl Pharmacol 238(3):209–214. https://doi.org/10.1016/j.taap.2009.01.029
Goering PL, Klassen CD (1984a) Tolerance to cadmium induced hepatotoxicity following cadmium pretreatment. Toxicol ApplPharmacol 74:308–313. https://doi.org/10.1016/0041-008X(84)90283-7
Friberg L, Kjellstrom T, Nordberg G, Piscator M (1979) “Cadmium” In handbook on the toxicology of metals (L.Friberg, G.F. Nordberg and V.B.Vonk Eds.), Elsevier/North Holland Biomedical Press. N.Y. 355–377
Soenen SJ, De M (2009) Cuyper, assessing cytotoxicity of (iron oxide based) nanoparticles: an overview of different methods exemplified with cationic magneto liposomes. Contrast Media Mol I 4(5):207–219. https://doi.org/10.1002/cmmi.282
Lynch I, Dawson KA (2008) Protein-nanoparticle interactions. Nano Today 3:40–47. https://doi.org/10.1016/S1748-0132(08)70014-8
Soenen SJ, Himmelreich U, Nuytten N, Pisanic TR, Ferrari A, De M (2010) Cuyper. Intracellular nanoparticle coating stability determines nanoparticle diagnostics efficacy and cell functionality. Small 6(19):2136–2145. https://doi.org/10.1002/smll.201000763
A.D. Lehmann, W.J. Parak, F. Zhang, Z. Ali, C. R€ocker, G.U. Nienhaus, P. Gehr, B. Rothen-Rutishause, Fluorescent magnetic hybrid nanoparticles induce a dose-dependent increase in proinflammatory response in lung cells in vitro correlated with intracellular localization, Small .6 (6) (2010) 753–762. https://doi.org/10.1002/smll.200901770
Waalkes MP, Wilson MJ, Poirier LA (1985) Reduced cadmium induced cytotoxicity in cultured liver cells following 5- azacytidine pretreatment. Toxicol Appl Pharmacol 81:250–257. https://doi.org/10.1016/0041-008x(85)90161-9
C.D, Klaassen, J. Liu, S. Choudhary, Metallothionein: an intracellular protein to protect against cadmium toxicity, Annu.Rev.Pharmacol. 39(1999) 267–294. https://doi.org/10.1146/annurev.pharmtox.39.1.267
Andrews GK (2000) Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem.Pharmacol. 59:95–104. https://doi.org/10.1016/S0006-2952(99)00301-9
Chen N, He Y, Su Y, Li X, Huang Q, Weng H, Zhang X, Tai R, Fan C (2012) The cytotoxicity of cadmium-based quantum dots. Biomaterials. 33:1238–1244
Rana SVS, Rastogi N (1998) Effect of cadmium on liver function in diabetic rats. Toxicol. Ind. Health 14:1–5. https://doi.org/10.1177/074823379801400306
Friberg L, Piscator M, Nordberg GF, Kjellstorm T (1974) Cadmium in the environment. CRC Press, Cleveland, Ohio
Dudley RE, Svoboda DJ, Klassen CD (1982) Acute exposure to cadmium causes severe liver injury in rats. Toxicol Appl Pharmacol 65:302–313. https://doi.org/10.1016/0041-008x(82)90013-8
Farber J (1979) Reactions of liver to injury: necrosis. In: Farber E, Fisher MM, eds. Toxic injury of the liver, Part A. New York: Marcel Dekker, 215–41
Lu L, Meiqing S, Qingzhao L, Hongmei Z, Alvarez PJJ, Huajie L, Wei C (2014) Genotoxicity and cytotoxicity of cadmium sulfide nanomaterials to mice: comparison between nanorods and nanodots. Environ Eng Sci 31(7):373–380. https://doi.org/10.1089/ees.2013.0417
M. Bednarski, , J. Dudek, L. Knustelska, J. Navinski, M. Sapa, G. Zygmunt, M Nowa K, , M. Luty-Bloch, M. Wojnicki, K. Fitzner, M. Tesiorowski, The influence of the route of administration of gold nanoparticles on their tissue distribution and basic biochemical parameters: in vivo studies,Pharmacol Rep. 67(2015) 405–409. https://doi.org/10.1016/j.pharep.2014.10.019
Rajan B, Satish S, Balakumar S, Devaki T (2015) Synthesis and dose interval dependent hepatotoxicity evaluation of intravenously administered polyethylene glycol-8000 coated ultra-small superparamagnetic iron oxide nanoparticle on Wistar rats. Environ Toxicol Pharmacol 39(2015):727–735. https://doi.org/10.1016/j.etap.2015.01.018
Gabor S, Anca Z, Bordas E (1978) Cadmium induced lipid peroxidation in kidney and testes. Effect of zinc and copper. Rev Roum Biochim 15:113–117
Waalkes MP, Wilson MJ, Poirier LA (1985) Reduced cadmium induced cytotoxicity in cultured liver cells following 5- azacytidine pretreatment. Toxicol Appl Pharmacol 81:250–257. https://doi.org/10.1016/0041-008x(85)90161-9
Sunderman FW Jr (1986) Metal and lipid peroxidation. Acta Pharmacol Toxicol 59(Suppl 7):248–255. https://doi.org/10.1111/j.1600-0773.1986.tb02755.x
Nel A, Xia T, Mädler L, Li N (2006) Toxic potential of materials at nanolevel. Science. 311:622–627
Xia T, Kovochich M, Liong M, Mädler L, Gilbert B, Shi H, Yeh JI, Zink JI, Nel AE (2008) Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS. Nano 2:2121–2134. https://doi.org/10.1021/nn800511k
T.R II. Pisanic, J. D. Blackwell, VI. Shubayev, R. R. Finones, S. Jin (2007) Nanotoxicity of iron oxide nanoparticle internalization in growing neurons, Biomaterials 28:2572–2581
Stroh A, Zimmer C, Gutzeit C, Jakstadt M, Marschinke F, Jung T, Pilgrimm H, Grune T (2004) Iron oxide particles for molecular magnetic resonance imaging cause transient oxidative stress in rat macrophages. Free Radic Biol Med 36(8):976–984. https://doi.org/10.1016/j.freeradbiomed.2004.01.016
Jain TK, Reddy MK, Morales MA, Leslie-Pelecky DL, Labhasetwar V (2008) Biodistribution, clearance and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol Pharm 5(2):316–327. https://doi.org/10.1021/mp7001285
Soto KF, Carrasco A, Powell TG, Garza KM, Murr LE (2005) Comparative in vitro cytotoxicity assessment of some manufactured nanoparticulate materials characterized by transmission electron microscopy. Nanoparticle Res 7:145–169. https://doi.org/10.1007/s11051-005-3473-1
Choi AO, Cho SJ, Desbarats J, Lovric J, Maysinger D (2007) Quantum dot-induced cell death involves Fas upregulation and lipid peroxidation in human neuroblastoma cells. J Nanotechnology 5:1–2. https://doi.org/10.1186/1477-3155-5-1
Stohs SJ, Bagchi D, Hassoun E, Bagchi M (2000) Oxidative mechanisms in the toxicity of chromium and cadmium ions. J Environ Pathol Toxicol Oncol 20:77–88
Sharma B, Singh S, Siddiqi NJ (2014) Biomedical implications of heavy metals induced imbalances in redox systems, Biomed Res Int. 640754. https://doi.org/10.1155/2014/640754
S.V.S. Rana , R. Singh, Species differences in glutathione- dependent enzymes in the liverand kidney of two fresh water fishes and their implications for cadmium toxicity. Ichthyol. Res .43(1996) 223–229. https://doi.org/10.1007/BF02347594
S.V.S. Rana and K.Taketa Eds. Oxidative stress and liver injury by environmental xenobiotics, In “Liver and Environmental Xenobiotcs”. Springer Verlag.pp 114–134
Donaldson K, Brown D, Clouter A, Duffin R, MacNee W, Renwick L, Tran L, Stone V (2002) The pulmonary toxicology of ultrafine particles. J Aerosol Med 15:213–220. https://doi.org/10.1089/089426802320282338
Akhtar MJ, Ahamed M, Kumar S, Khan MM, Ahmed J, Alrokayan SA (2012) Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int J Nanomedicine 7:845–857. https://doi.org/10.2147/ijn.s29129
Colucci AV, Winge D, Krasno MD (1975) Cadmium accumulation in rat liver. Arch Environ Health 30:153–157. https://doi.org/10.1080/00039896.1975.10666665
Stowe HD, Wilson M, Goyer RA (1972) Clinical and pharmacological effects of oral cadmium toxicity in rabbits. Arch.Pathol. 94:389–405
Hoffman EO, Cook JA, Diluzio NR, Coover JA (1975) The effects of acute cadmium administration in the liver and kidney of rat. Light and Electron microscopic studies. Lab Investig 32:655–664
Dudley RE, Svoboda DJ, Klassen CD (1984) Time course of cadmium induced ultrastructural changes in rat liver. Toxicol Appl Pharmacol 76(1):15–160. https://doi.org/10.1016/0041-008X(84)90038-3
M. Wang, J. Wang, H. Sun, S. Han, S. Feug, L. Shi, P. Meng, J. Li, P. Huang, , Z. Sun, Time dependent toxicity of cadmium chloride quantum dots on liver and kidney of mice: histopathological changes with elevated free cadmium ions and hydroxyl radicals, Int J Nanomedicine 11(2016), 2319–2328. https://doi.org/10.2147/IJN.S103489
Liu L, Sun M, Li Q, Zhang H, Alvarez PJJ, Liu H, Chen W (2014) Genotoxicity and cytotoxicity of cadmium sulfide nanomaterials to mice: comparison between nanoraods and nanodots. Environ Eng Sci 31:373–380. https://doi.org/10.1089/ees.2013.0417
Xiu ZM, Zhang QB, Puppala HL, Colvin VL, Alveraj PJ (2012) Negligible particle specific antibacterial activity of silver nanoparticles. Nano Lett 12:4271–4275. https://doi.org/10.1021/nl301934w
Sina JF, Chin B (1978) Cadmium modification of nucleolar ultrastructure and RNA synthesis in Physarumpolycephalum. Toxicol Appl Pharmacol 43:449–459. https://doi.org/10.1016/S0041-008X(78)80004-0
Puvian E, Lange M (1980) Functional significance of perichromatin granule accumulation induced by cadmium chloride in isolated liver cells. Exp Cell Res 128:47–58. https://doi.org/10.1016/0014-4827(80)90385-7
Gamulin S, Car N, Narancsik P (1982) Effects of cadmium on polyribosome structure and function in mouse liver. Experimenta. 33:1144–1145. https://doi.org/10.1007/bf01922292
Verma A, Stellacci F (2006) Effect of surface properties on nanoparticle cell interactions. Small 6(1):12–21. https://doi.org/10.1002/smll.200901158
Cho SJ, Maysinger D, Jain M, Roder B, Hackbarth S, Winnik FM (2007) Long term exposure to CdTe quantum dots causes functional impairments in live cells. Langmuir. 23:1974–1980. https://doi.org/10.1021/la060093j
P.X. Petit , H. Lecocur, E. Zorn, , C. Daughet, B. Mignotte, M. L.Gongeon, Alterations in mitochondrial structure and function are early events of dexamethasone induced thymocyte apoptosis, J Cell Biol 130(1995) 157–167. https://doi.org/10.1083/jcb.130.1.157
Armstrong JS (2006) Mitochondrial membrane permeabilization: the sine qua non for cell death. Bioassays. 28:253–260. https://doi.org/10.1002/bies.20370
Yeh JH, Huang CC, Yeh MV, Wang JS, Lee JK, Jan CR (2009) Cadmium induced cytosolic Ca2+ elevation and subsequent apoptosis in renal tubular cells. Basic Clin Pharmacol Toxicol 104:345–351
Brand MD, Nicholls DG (2011) Assessing mitochondrial dysfunction in cells. Biochem J 435:297–312. https://doi.org/10.1042/BJ20110162
Acknowledgments
The authors thank Director, Sophisticated Analytical Instrument Center, Punjab University, Chandigarh for FE-SEM, TEM, and EDAX. Director, Sophisticated Instrument facility of Indian Institute of Technology, Roorkeeis, is acknowledged for DLS and zeta potential. We are grateful to the In-charge, Electron Microscope Facility, All India Institute of Medical Sciences, New Delhi, for extending all possible help in TEM for ultrastructural studies of tissue.
Funding
The Department of Science and Technology, New Delhi, provided financial support (SR/FT/LS-46/2011) KR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rana, K., Verma, Y. & Rana, S.V.S. Possible Mechanisms of Liver Injury Induced by Cadmium Sulfide Nanoparticles in Rat. Biol Trace Elem Res 199, 216–226 (2021). https://doi.org/10.1007/s12011-020-02128-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02128-5