Abstract
Intracellular microsporidian Nosema mylitta infects Indian wild silkworm Antheraea mylitta causing pebrine disease. Genetic structure and phylogeny of N. mylitta are analysed using nucleotide variability in 5S ribosomal DNA and intergenic spacer (IGS) sequence from 20 isolates collected from Southern, Northern and Central regions of Jharkhand State. Nucleotide diversity (π) and genetic differentiation Gst were highest in the Central isolates whereas lowest in the North. Among the isolates, absence of nucleotides, transitions and transversions were observed. Haplotyping showed nucleotide variability at 83 positions in IGS and 13 positions in 5S rDNA. Haplotype-based genetic differentiation was 0.96 to 0.97 whereas nucleotide sequence-based genetic differentiation was higher (Ks = 22.29) between Southern and Central isolates. Bottleneck analysis showed negative value for Tajima’s D and other summary statistics revealing induction of loss of rare alleles and population explosion. From IGS, 17 ancestral sequences were inferred by Network algorithm. Core of nine closely related nodes having ancient nucleotides and peripheral nodes with highly divergent nucleotides were derived. Most diverged peripheral haplotype was Bero (H11) from the Central region whereas Deoghar (H3) of the Northern region diverged early. Phylogeny of N. mylitta grouped Southern and Northern isolates together revealed weak phylogenetic signal for these locations. Phylogeny of N. mylitta with Nosema sp. infecting other lepidopterans clustered N. mylitta isolates with N. antheraea and N. philosamiae of China indicating genetic similarity whereas other species were dissimilar showing diversity irrespective of country of origin.






Similar content being viewed by others
References
Alam K, Bhattacharya D, Chowdhury T, Saha S, Kar P (2018) Biodiversity status and conservational requirements of tropical tasar (Antheraea mylitta D)—a review. Ecol Environ Conserv 24:1887–1894
Andersson SGE, Kurland CG (1995) Genomic evolution drives the evolution of the translation system. Biochem Cell Biol 73:775–787
Andersson SGE, Kurland CG (1998) Reductive evolution of resident genomes. Trends Microbiol 6:263–268
Armbruster G, Pfenninger M (2003) Simulated bottlenecks and loss of rare alleles: implications on the conservation genetics of two gastropod species. J Nat Conserv 11:77–81
Bandelt HJ, Forster P, Rohl A (1999) Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16:37–48
Bush SE, Villa SM, Altuna JC, Johnson KP, Shapiro MD, Clayton DH (2019) Host defense triggers rapid adaptive radiation in experimentally evolving parasites. Evol Lett 3:120–128
Cali A, Takvorian PM (2014) Developmental morphology and life cycles of the microsporidia. In: Weiss LM, Becnel JJ (eds) Microsporidia: pathogens of opportunity. Wiley-Blackwell, Oxford, pp 71–133
Chakrabarti S, Manna B (2006) Three new species from Nosema-like isolates of three non-mulberry silkworms in Assam: light, scanning and transmission electron microscopy. J Parasit Dis 30:125–133
Chakraborty S, Muthulakshmi M, Vardhini D, Jayaprakash P, Nagaraju J, Arunkumar KP (2015) Genetic analysis of Indian tasar silkmoth (Antheraea mylitta) populations. Sci Rep 5:15728. https://doi.org/10.1038/srep15728
Cuomo CA, Desjardins CA, Bakowski MA, Goldberg J, Ma AT, Becnel JJ, Didier ES, Fan L, Heiman DI, Levin JZ, Young S, Zeng Q, Troemel ER (2012) Microsporidian genome analysis reveals evolutionary strategies for obligate intracellular growth. Genome Res 22:2478–2488
Dean P, Sendra KM, Williams TA, Watson AK, Major P, Nakjang S, Kozhevnikova E, Goldberg AV, Kunji ERS, Hirt RP, Embley TM (2018) Transporter gene acquisition and innovation in the evolution of Microsporidia intracellular parasites. Nat Commun. https://doi.org/10.1038/s41467-018-03923-4
Dobrindt U, Hacker J (2001) Whole genome plasticity in pathogenic bacteria. Curr Opin Microbiol 4:550–557
Dover GA (1982) Molecular drive: a cohesive mode of species evolution. Nature 299:111–117
Dussaubat C, Sagastume S, Moracho TG, Botias C, Palencia PG, Hernandez RM, Conte YL, Higes M (2012) Comparative study of Nosema ceranae (Microsporidia) isolates from two different geographic origins. Vet Microbiol 162:670–767
Faith D (1994) Phylogenetic pattern and the quantification of organismal biodiversity. Phil Trans R Soc Lond B Biol Sci 345:45–58
Fay JC, Wu C-I (2000) Hitchhiking under positive Darwinian selection. Genetics 155:1405–1413
Flohr RCE, Blom CJ, Rainey PB, Beaumont HJE (2013) Founder niche constrains evolutionary adaptive radiation. Proc Nat Acad Sci USA 110:20663–20668
Fournier PE, Zhu Y, Ogata H, Raoult D (2004) Use of highly variable intergenic spacer sequences for multispacer typing of Rickettsia conorii strains. J Clin Microbiol 42:5757–5766
Fu Y-X, Li W-H (1993) Statistical tests of neutrality of mutations. Genetics 133:693–709
Galián JA, Marcela R, Josep AR (2014) Partial sequence homogenization in the 5S multigene families may generate sequence chimeras and spurious results in phylogenetic reconstructions. Syst Biol 63:219–230
Gatehouse HS, Malone LA (1998) The ribosomal RNA gene region of Nosema apis (Microspora): DNA sequence for small and large subunit rRNA genes and evidence of a large tandem repeat unit size. J Invertebr Pathol 71:97–105
Gorokhova E, Dowling TE, Weider LJ, Crease TJ, Elser JJ (2002) Functional and ecological significance of rDNA intergenic spacer variation in a clonal organism under divergent selection for production rate. Proc R Soc Lond B 269:2373–2379. https://doi.org/10.1098/rspb.2002.2145
Guo Z, Shi W, Zhang L, Wang G (2004) Behavioral and morphological indices for phase transformation of oriental migratory locust Locusta migratoria manilensis. J Appl Ecol 15:859–862
Gupta SK, Hossain Z, Nanu MM, Mondal K (2016) Impact of microsporidian infection on growth and development of silkworm Bombyx mori L. (Lepidoptera: Bombycidae). ANRES 50:388–395
Hall TA (1999) Bioedit: a user-friendly biological alignment editor and analysis programme for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Han B, Weiss LM (2017) Microsporidia: obligate intracellular pathogens within the Fungal kingdom. Microbiol Spectr. https://doi.org/10.1128/microbiolspec.FUNK-0018-2016
Hassan W, Nath BS (2015) Genetic characterisation of microsporidia infecting Indian tasar silkworm, Antheraea mylitta, using morphology and molecular tools. Folia Parasitol 62:034
Hassan W, Nath BS, Murthy GN (2016) Comparative phylogenetic analysis of intergenic spacers and small subunit rRNA gene sequences of two microsporidian isolates from Antheraea myllita. Afr J Biotech 15:2678–2686
Hatakeyama Y, Bansal AK, Iwano H, Kawakami Y, Ishihara R (2000) Characterisation of SSU rRNA sequence of a new microsporidium Nosema sp. (Nosematidae: Microsporidia) isolated from Antheraea mylitta Drury (Lepidoptera: Saturnidae) in India. Ind J Seric 39:131–134
Higgins D, Thompson J, Gibson T, Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Hillis DM, Dixon MT (1991) Ribosomal DNA: molecular evolution and phylogenetic inference. Quart Rev Biol 66(4):411–453
Hodges SA, Derieg NJ (2009) Adaptive radiations: from field to genomic studies. Proc Nat Acad Sci USA 106:9947–9954
Huang WF, Tsai SJ, Lo CF, Yamane S, Wang CH (2004) The novel organization and complete sequence of the ribosomal RNA gene of Nosema bombycis. Fungal Genet Biol 41:473–481
Huang WF, Bocquet M, Lee KC, Sung IH, Jiang JH, Chen YW, Wang CH (2008) The comparison of rDNA spacer regions of Nosema ceranae isolates from different hosts and locations. J Invertbr Pathol 97:9–13
Hudson RR, Montgomery S, Wayne PM (1992) Estimation of levels of gene flow from DNA sequence data. Genetics 132:583–589
Hungund SP, Pradeep AR, Pooja M, Sagar C, Mishra RK (2019) Cellular defence and innate immunity in the larval ovarian disc and differentiated ovariole of the silkworm Bombyx mori induced by microsporidian infection. Invertebr Reprod Dev. https://doi.org/10.1080/07924259.2019.1669727
Hyten DL, Song Q, Zhu Y, Choi I-Y, Nelson RL, Costa JM, Specht JE, Shoemaker RC, Cregan PB (2006) Impacts of genetic bottlenecks on soybean genome diversity. Proc Nat Acad Sci USA 103:16666–16671
Ironside JE (2013) Diversity and recombination of dispersed ribosomal DNA and protein coding genes in microsporidia. PLoS ONE 8(2):e55878. https://doi.org/10.1371/journal.pone.0055878
Jukes T, Cantor CR (1969) Evolution of protein molecules. In: Munro HN (ed) Mammalian protein metabolism. Academic Press, New York, pp 21–132
Katoh M, Katoh M (2003) FNBP2 gene on human chromosome 1q32.1 encodes ARHGAP family protein with FCH, FBH, RhoGAP and SH3 domains. Int J Mol Med 11:791–797
Keeling PJ, McFadden GI (1998) Origins of microsporidia. Trends Microbiol 6:19–23
Kellis M, Birren BW, Lander ES (2004) Proof and evolutionary analysis of ancient genome duplication in the yeast Saccharomyces cerevisiae. Nature 428:617–624
Kolaczkowski B, Thornton J (2004) Performance of maximum parsimony and likelihood phylogenetics when evolution is heterogeneous. Nature 431:980–984
Lee SC, Weiss LM, Heitman J (2009) Generation of genetic diversity in microsporidia via sexual reproduction and horizontal gene transfer. Commun Integr Biol 2:414–417
Lee SC, Heitman J, Ironside JE (2014) Sex and the microsporidia. In: Weiss LM, Becnel JJ (eds) Microsporidia: pathogens of opportunity. Wiley, Chichester, pp 231–243
Leski TA, Stockelman MG, Moses LM, Park M, Stenger DA, Ansumana R, Bausch DG, Lin B (2015) Sequence variability and geographic distribution of Lassa Virus, Sierra Leone. Emerg Infect Dis 21:609–618
Li JL, Chen WF, Wu J, Peng WJ, An JD, Schmid-Hempel P, Schmid-Hempel R (2012) Diversity of Nosema associated with bumblebees (Bombus spp.) from China. Int J Parasitol 42:49–61
Maddox JV, Mcmanus ML, Solter LF (1998) Microsporidia affecting forest Lepidoptera. In: Population dynamics, impacts, and integrated management of forest defoliating insects. USDA Forest Service General Technical Report NE-247, pp 187–197
Michonneau F (2016) Using GMYC for species delination. Zenodo. https://doi.org/10.5281/zenodo.838260
Mishra CSK, Nayak BK, Dash MC (1992) Larval mortality of Indian tasar silkworm (Antheraea mylitta) (Saturniidae) due to pébrine infection. J Lepid Soc 46:106–109
Nath BS, Gupta SK, Bajpai AK (2012) Molecular characterization and phylogenetic relationships among microsporidian isolates infecting silkworm, Bombyx mori using small subunit rRNA (SSU-rRNA) gene sequence analysis. Acta Parasitol 57:342–353
Nei M (1973) Analysis of gene diversity in subdivided populations. Proc Natl Acad Sci USA 70:3321–3323
Nielsen R, Beaumont MA (2009) Statistical inferences in phylogeography. Mol Ecol 18:1034–1047
Pan G, Xu J, Li T, Xia Q et al (2013) Comparative genomics of parasitic silkworm microsporidia reveal an association between genome expansion and host adaptation. BMC Genomics 14:186
Pavlopoulos GA, Soldatos TG, Barbosa-Silva A, Schneider R (2010) A reference guide for tree analysis and visualization. BioData Mining 3:1
Pecinka A, Fang W, Rehmsmeier M, Levy AA, Scheid OM (2011) Polyploidization increases meiotic recombination frequency in Arabidopsis. BMC Biol 9:24. https://doi.org/10.1186/1741-7007-9-24
Pelin A, Selman M, Aris-Brosou S, Farinelli L, Corradi N (2015) Genome analyses suggest the presence of polyploidy and recent human-driven expansions in eight global populations of the honeybee pathogen Nosema ceranae. Environ Microbiol 17:4443–4458
Pinho C, Harris DJ, Ferrand N (2008) Non-equilibrium estimates of gene flow inferred from nuclear genealogies suggest that Iberian and North African wall lizards (Podarcis spp.) are an assemblage of incipient species. BMC Evol Biol. https://doi.org/10.1186/1471-2148-8-63
Pradeep AR, Awasthi AK, Singh CK, Anuradha HJ, Rao CG, Vijayaprakash NB (2011) Genetic evaluation of eri silkworm Samia cynthia ricini: ISSR loci specific to high and low altitude regimes and quantitative attributes. J Appl Genet 52:345–353
Przeworski M (2002) The signature of positive selection at randomly chosen Loci. Genetics 160:1179–1189
Rao SN, Muthulakshmi M, Kanginakudru S, Nagaraju J (2004) Phylogenetic relationships of three new microsporidian isolates from the silkworm, Bombyx mori. J Invertbr Pathol 86:87–95
Rozas J, Ferrer-Mata A, Sánchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, Sánchez-Gracia A (2017) DnaSP 6: DNA sequence polymorphism analysis of large datasets. Mol Biol Evol 34:3299–3302
Sano A, Tachida H (2005) Gene genealogy and properties of test statistics of neutrality under population growth. Genetics 169:1687–1697
Santin M, Fayer R (2009) Enterocytozoon bieneusi genotype nomenclature based on the internal transcribed spacer sequence: a Consensus. J Eukaryot Microbiol 56:34–38
Storz JF, Kelly JK (2008) Effects of spatially varying selection on nucleotide diversity and linkage disequilibrium: insights from deer mouse globin genes. Genetics 180:367–379
Syvanen AC, Amiri H, Jamal A, Andersson SG, Kurland CG (1996) A chimeric disposition of the elongation factor genes in Rickettsia prowazekii. J Bacteriol 178:6192–6199
Tajima F (1983) Evolutionary relationship of DNA sequences in finite populations. Genetics 105:437–460
Tajima F (1989) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123:585–595
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10:512–526
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolution Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729
Tan SQ, Zhang KQ, Chen HX, Ge Y, Ji R, Shi WP (2015) The mechanism for microsporidian parasite suppression of the hindgut bacteria of the migratory locust Locusta migratoria manilensis. Sci Rep 5:17365. https://doi.org/10.1038/srep17365
Tanada Y, Kaya HK (1993) Protozoan infections: Apicomplexa, microspora. In: Tanada Y, Kaya HK (eds) Insect pathology. Academic Press, San Diego, pp 414–458
Tsai SJ, Lo CF, Soichi Y, Wang CH (2003) The characterization of microsporidian isolates (Nosematidae: Nosema) from five important lepidopteran pests in Taiwan. J Invertbr Pathol 83:51–59
Tsai SJ, Huang WF, Wang CH (2005) Complete sequence and gene organization of the Nosema spodopterae rRNA Gene. J Eukaryot Microbiol 52:52–54
Tsai YC, Solter LF, Wang CY, Fan HS, Chang CC, Wang CH (2009) Morphological and molecular studies of a microsporidium (Nosema sp.) isolated from the three-spot grass yellow butterfly, Eurema blanda arsakia (Lepidoptera: Pieridae). J Invertbr Pathol 100:85–93
Undeen AH, Alger NE (1971) A density gradient method for fractionating microsporidian spores. J Invertbr Pathol 18:419–420
Undeen AH, Cockburn AF (1989) The extraction of DNA from microsporidia spores. J Invertbr Pathol 54:132–133
Vijayan K, Nair CV, Kar P, Mohandas TP, Saratchandra B, Urs SR (2005) Genetic variability within and among three ecoraces of the tasar silkworm Antheraea mylitta Drury as revealed by ISSR and RAPD Markers. Int J Indust Entomol 10:51–59
Volkov RA, Irina IP, Nikolai VB, Marta H-B, Jolanta M, Vera H (2017) Evolutional dynamics of 45S and 5S ribosomal DNA in ancient allohexaploid Atropa belladonna. BMC Plant Biol 17:21. https://doi.org/10.1186/s12870-017-0978-6
Wagner A (2007) Rapid detection of positive selection in genes and genomes through variation clusters. Genetics 176:2451–2463
Wall C, Perry JN (1987) Range of action of moth sex attractant sources. Entomol exp appl 44:5–14
Wang LL, Chen KP, Yao Q (2006) The sequence and phylogenetic analysis of the ribosomal RNA gene and the ITS region of Nosema antheraeae. Sci Agric Sin 39:1674–1679
Weiss LM, Becnel JJ (2014) Microsporidia: Pathogens of Opportunity. Wiley-Blackwell; Hoboken, NJ
Wittner M, Weiss LM (1999) The microsporidia and microsporidiosis. American Society for Microbiology, Washington
Yoder JB, Clancey E, Des RS, Eastman JM, Gentry L, Godsoe W, Hagey TJ, Jochimsen D, Oswald BP, Robertson J, Sarver BAJ, Schenk JJ, Spear SF, Harmon LJ (2010) Ecological opportunity and the origin of adaptive radiations. J Evol Biol 23:1581–1596
Acknowledgements
The authors thank anonymous referees for critical comments that improved the manuscript and Central Silk Board, Bangalore, India, for laboratory facilities. Dr. Wazid Hassan is a recipient of Research Associateship from Council of Scientific and Industrial Research (CSIR), Government of India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This study included research on silkworms and microsporidian parasites. No study on humans is conducted. All procedures are in compliance with ethical standards.
Additional information
Handling editor: Alexander Platt.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file1 (TIF 897 kb)
Supplementary Figure 1: (A) Scanning electron microscopy of the N. mylitta spores showing elongated oval shape and smooth surface. Size of the spores ranged from 3.8 - 5.1 µm in length and 2.6 - 3.3 µm in width. (B) PCR profile showed amplification of SSU-IGS-5S rDNA sequence from the genomic DNA extracted from 20 isolates of N. mylitta (1-20). M- Molecular weight marker (Promega)
Supplementary file2 (TIF 1545 kb)
Supplementary Figure 2: Multiple sequence alignment of 5S rDNA sequences from 20 isolates of N. mylitta by Clustal W showing gaps, transitions and transversions. The details of accessions are given in Table 1. * indicates conserved nucleotides.
Supplementary file3 (TIF 4074 kb)
Supplementary Figure 3: Multiple sequence alignment of IGS sequence from 20 isolates of N. mylitta by Clustal W showing gaps, transitions and transversions. The details of accessions are given in Table 1. * indicates conserved nucleotides.
Supplementary file4 (TIF 575 kb)
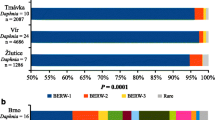
Supplementary Figure 4: Distribution of informative variable nucleotides at 13 positions in 5S rDNA sequences of 20 isolates of N. mylitta realised after haplotyping using DnaSP software. Ten haplotypes were created out of which haplotypes 3 formed from 11 isolates. Details of haplotypes are given in right column.
Supplementary file5 (TIF 165 kb)
Supplementary Figure 5: Phylogram realised from nucleotide sequences of IGS region of 20 N. mylitta isolates and 20 Nosema species infecting various insects and a microsporidian genus Vairimorpha sp (as an out-group). The sequences were retrieved from the NCBI database (Supplementary Table 1) and aligned using Clustal W program. The unrooted tree was constructed using Neighbor Joining method with branch and bound option and with 1000 bootstrap replicates as given in the MEGA program. All N. mylitta isolates grouped together along with N. antheraea and N. philosamiae. Other Nosema species were grouped into two clusters and Vairimorpha separated as an out group. Details of accessions and host insects are given in Supplementary Table 1.
Supplementary file6 (TIF 147 kb)
Supplementary Figure 6: Phylogram realised from nucleotide sequences of 5S rDNA region of 20 N. mylitta isolates and 20 Nosema species infecting various insects and a microsporidian genus Vairimorpha sp (as an out-group). The sequences were retrieved from the NCBI database (Supplementary Table 1) and aligned using Clustal W program. The unrooted tree was constructed using Neighbor Joining method with branch and bound option and with 1000 bootstrap replicates as given in the MEGA program. All the N. mylitta isolates and other Nosema species grouped together into single cluster and Vairimorpha separated as an outgroup. Details of accessions and host insects are given in Supplementary Table 1.
Rights and permissions
About this article
Cite this article
Hassan, W., Nath, B.S., Ponnuvel, K.M. et al. Evolutionary Diversity in the Intracellular Microsporidian Parasite Nosema sp. Infecting Wild Silkworm Revealed by IGS Nucleotide Sequence Diversity. J Mol Evol 88, 345–360 (2020). https://doi.org/10.1007/s00239-020-09936-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00239-020-09936-2