New Crystal Forms for Biologically Active Compounds. Part 2: Anastrozole as N-Substituted 1,2,4-Triazole in Halogen Bonding and Lp-π Interactions with 1,4-Diiodotetrafluorobenzene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. X-ray Structure Determination
2.3. Powder X-ray Diffraction Experiments
2.4. Computational Details
3. Results and Discussion
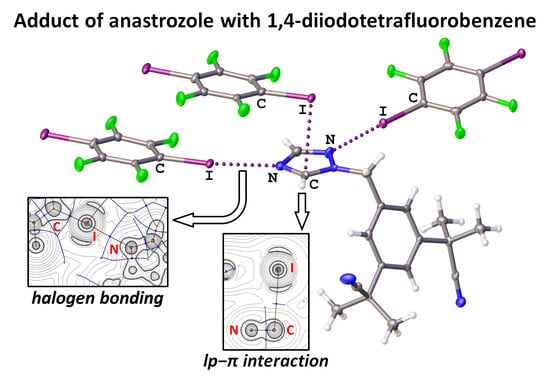
3.1. Halogen Bonding in ASZ·1.5(1,4-FIB)
3.2. Lone-Pair∙∙∙π Interactions in ASZ·1.5(1,4-FIB)
3.3. Hydrogen Bonding in ASZ·1.5(1,4-FIB)
3.4. Theoretical Study of Different Non-covalent Interactions in ASZ·1.5(1,4-FIB)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stahl, P.H.; Wermiuth, C.G.E. Handbook of Pharmaceutical Salts Properties, Selection and Use; Verlag Helvetica Chimica Acta: Zürich, Switzerland, 2002. [Google Scholar]
- Trask, A.V. An overview of pharmaceutical cocrystals as intellectual property. Mol. Pharm. 2007, 4, 301–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trask, A.V.; Motherwell, W.D.S.; Jones, W. Pharmaceutical cocrystallization: Engineering a remedy for caffeine hydration. Cryst. Growth Des. 2005, 5, 1013–1021. [Google Scholar] [CrossRef]
- Karki, S.; Friščić, T.; Jones, W.; Motherwell, W.D.S. Screening for pharmaceutical cocrystal hydrates via neat and liquid-assisted grinding. Mol. Pharm. 2007, 4, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Merkens, C.; Pan, F.F.; Englert, U. 3-(4-Pyridyl)-2,4-pentanedione—A bridge between coordinative, halogen, and hydrogen bonds. CrystEngComm 2013, 15, 8153–8158. [Google Scholar] [CrossRef]
- Cinčić, D.; Friščić, T.; Jones, W. Structural Equivalence of Br and I Halogen Bonds: A Route to Isostructural Materials with Controllable Properties. Chem. Mater. 2008, 20, 6623–6626. [Google Scholar] [CrossRef]
- Cinčić, D.; Friščić, T.; Jones, W. A cocrystallisation-based strategy to construct isostructural solids. New J. Chem. 2008, 32, 1776–1781. [Google Scholar] [CrossRef]
- Bushuyev, O.S.; Tan, D.; Barrett, C.J.; Friščić, T. Fluorinated azobenzenes with highly strained geometries for halogen bond-driven self-assembly in the solid state. CrystEngComm 2015, 17, 73–80. [Google Scholar] [CrossRef]
- Cinčić, D.; Friščić, T. Synthesis of an extended halogen-bonded metal-organic structure in a one-pot mechanochemical reaction that combines covalent bonding, coordination chemistry and supramolecular synthesis. CrystEngComm 2014, 16, 10169–10172. [Google Scholar] [CrossRef]
- Troff, R.W.; Makela, T.; Topic, F.; Valkonen, A.; Raatikainen, K.; Rissanen, K. Alternative Motifs for Halogen Bonding. Eur. J. Org. Chem. 2013, 2013, 1617–1637. [Google Scholar] [CrossRef]
- Cinčić, D.; Friščić, T.; Jones, W. A stepwise mechanism for the mechanochemical synthesis of halogen-bonded cocrystal architectures. J. Am. Chem. Soc. 2008, 130, 7524–7525. [Google Scholar] [CrossRef]
- Carletta, A.; Spinelli, F.; d’Agostino, S.; Ventura, B.; Chierotti, M.R.; Gobetto, R.; Wouters, J.; Grepioni, F. Halogen-Bond Effects on the Thermo- and Photochromic Behaviour of Anil-Based Molecular Co-crystals. Chem.: Eur. J. 2017, 23, 5317–5329. [Google Scholar] [CrossRef] [PubMed]
- Stilinović, V.; Horvat, G.; Hrenar, T.; Nemec, V.; Cinčić, D. Halogen and Hydrogen Bonding between (N-Halogeno)-succinimides and Pyridine Derivatives in Solution, the Solid State and In Silico. Chem. Eur. J. 2017, 23, 5244–5257. [Google Scholar] [CrossRef] [PubMed]
- Kryukova, M.A.; Sapegin, A.V.; Novikov, A.S.; Krasavin, M.; Ivanov, D.M. New Crystal Forms for Biologically Active Compounds. Part 1: Noncovalent Interactions in Adducts of Nevirapine with XB Donors. Crystals 2019, 9, 71. [Google Scholar] [CrossRef] [Green Version]
- Kryukova, M.A.; Sapegin, A.V.; Novikov, A.S.; Krasavin, M.; Ivanov, D.M. Non-covalent interactions observed in nevirapinium pentaiodide hydrate which include the rare I4–I− ⋯O=C halogen bonding. Z. Kristallogr. Cryst. Mater. 2019, 234, 101–108. [Google Scholar] [CrossRef]
- Wiseman, L.R.; Adkins, J.C. Anastrozole—A review of its use in the management of postmenopausal women with advanced breast cancer. Drugs Aging 1998, 13, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Augusto, T.V.; Correia-da-Silva, G.; Rodrigues, C.M.P.; Teixeira, N.; Amaral, C. Acquired resistance to aromatase inhibitors: Where we stand! Endocr.-Relat. Cancer 2018, 25, R283–R301. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, R.J.; Reid, G.D.; Koch, J.; Deans, R.; Ledger, W.L.; Friedlander, M.; Gilchrist, R.B.; Walters, K.A.; Abbott, J.A. The safety and efficacy of controlled ovarian hyperstimulation for fertility preservation in women with early breast cancer: A systematic review. Hum. Reprod. 2017, 32, 1033–1045. [Google Scholar] [CrossRef] [Green Version]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Definition of the hydrogen bond (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 1637–1641. [Google Scholar] [CrossRef]
- Tang, G.P.; Gu, J.M. 2-[3-(2-cyano-2-propyl)-5-(1,2,4-triazol-1-yl)phenyl]-2-methylpropiononitrile. Acta Cryst. E 2005, 61, O2330–O2331. [Google Scholar] [CrossRef]
- Capucci, D.; Balestri, D.; Mazzeo, P.P.; Pelagatti, P.; Rubini, K.; Bacchi, A. Liquid Nicotine Tamed in Solid Forms by Cocrystallization. Cryst. Growth Des. 2017, 17, 4958–4964. [Google Scholar] [CrossRef]
- Choquesillo-Lazarte, D.; Nemec, V.; Cinčić, D. Halogen bonded cocrystals of active pharmaceutical ingredients: Pyrazinamide, lidocaine and pentoxifylline in combination with haloperfluorinated compounds. CrystEngComm 2017, 19, 5293–5299. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT - Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Agilent Technologies Ltd. CrysAlisPro; Version 1.171.136.120 (release 127-106-2012); Agilent Technologies Ltd.: Santa Clara, CA, USA, 2012. [Google Scholar]
- Chai, J.D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, EM64L-G09RevB. 01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Barros, C.L.; de Oliveira, P.J.P.; Jorge, F.E.; Canal Neto, A.; Campos, M. Gaussian basis set of double zeta quality for atoms Rb through Xe: Application in non-relativistic and relativistic calculations of atomic and molecular properties. Mol. Phys. 2010, 108, 1965–1972. [Google Scholar] [CrossRef]
- de Berrêdo, R.C.; Jorge, F.E. All-electron double zeta basis sets for platinum: Estimating scalar relativistic effects on platinum(II) anticancer drugs. J. Mol. Struct. THEOCHEM 2010, 961, 107–112. [Google Scholar] [CrossRef]
- Jorge, F.E.; Canal Neto, A.; Camiletti, G.G.; Machado, S.F. Contracted Gaussian basis sets for Douglas–Kroll–Hess calculations: Estimating scalar relativistic effects of some atomic and molecular properties. J. Chem. Phys. 2009, 130, 064108. [Google Scholar] [CrossRef]
- Neto, A.C.; Jorge, F.E. All-electron double zeta basis sets for the most fifth-row atoms: Application in DFT spectroscopic constant calculations. Chem. Phys. Lett. 2013, 582, 158–162. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Oh, S.Y.; Nickels, C.W.; Garcia, F.; Jones, W.; Friščić, T. Switching between halogen- and hydrogen-bonding in stoichiometric variations of a cocrystal of a phosphine oxide. CrystEngComm 2012, 14, 6110–6114. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Steiner, T. The hydrogen bond in the solid state. Angew. Chem.-Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Pandiyan, B.V.; Deepa, P.; Kolandaivel, P. How do halogen bonds (S–O⋯I, N–O⋯I and C–O⋯I) and halogen-halogen contacts (C–I⋯I–C, C–F⋯F–C) subsist in crystal structures? A quantum chemical insight. J. Mol. Model. 2017, 23, 16. [Google Scholar] [CrossRef]
- Li, L.L.; Liu, Z.F.; Wu, W.X.; Jin, W.J. Cocrystals with tunable luminescence colour self-assembled by a predictable method. Acta Crystallogr. Sect. B 2018, 74, 610–617. [Google Scholar] [CrossRef]
- DeHaven, B.A.; Chen, A.L.; Shimizu, E.A.; Salpage, S.R.; Smith, M.D.; Shimizu, L.S. Synergistic effects of hydrogen and halogen bonding in co-crystals of dipyridylureas and diiodotetrafluorobenzenes. Supramol. Chem. 2018, 30, 315–327. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding and other s-hole interactions: A perspective. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. [Google Scholar] [CrossRef]
- Murray, J.S.; Politzer, P. Interaction and Polarization Energy Relationships in s-Hole and p-Hole Bonding. Crystals 2020, 10, 76. [Google Scholar] [CrossRef] [Green Version]
- Bondi, A. Van der Waals volumes + radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Zhang, K.L.; Hou, C.T.; Song, J.J.; Deng, Y.; Li, L.; Ng, S.W.; Diao, G.W. Temperature and auxiliary ligand-controlled supramolecular assembly in a series of Zn(II)-organic frameworks: Syntheses, structures and properties. CrystEngComm 2012, 14, 590–600. [Google Scholar] [CrossRef]
- Zhang, K.L.; Chang, Y.; Ng, S.W. Preparation and characterization of two supramolecular complexes with 5-amino-2,4,6-triiodoisophthalic acid under N-donor auxiliary ligand intervention. Inorg. Chim. Acta 2011, 368, 49–57. [Google Scholar] [CrossRef]
- Wang, J.W.; Chen, C.; Li, Y.J.; Luo, Y.H.; Sun, B.W. Halogen-bonding contacts determining the crystal structure and fluorescence properties of organic salts. New J. Chem. 2017, 41, 9444–9452. [Google Scholar] [CrossRef]
- Rode, N.D.; Sonawane, A.D.; Garud, D.R.; Joshi, R.R.; Joshi, R.A.; Likhite, A.P. First regioselective iodocyclization reaction of 3-aryl-5-(prop-2-ynylthio)-1H-1,2,4-triazoles. Tetrahedron Lett. 2015, 56, 5140–5144. [Google Scholar] [CrossRef]
- Luo, Y.H.; Sun, Y.; Liu, Q.L.; Yang, L.J.; Wen, G.J.; Wang, M.X.; Sun, B.W. Influence of Halogen Atoms on Spin-Crossover Properties of 1,2,4-Triazole-Based 1D Iron(II) Polymers. ChemistrySelect 2016, 1, 3879–3884. [Google Scholar] [CrossRef]
- Xiong, H.-P.; Gao, S.-H.; Li, C.-T.; Wu, Z.-J. (2RS)-2-(2,4-Difluorophenyl)-1-[(4-iodobenzyl)(methyl)amino]-3-(1H-1,2,4-triazol-1-yl)propan-2-ol. Acta Cryst. E 2012, 68, o2447–o2448. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Chi, Y.; Mang, X.Y.; Zhang, G.Q.; Zhang, Y.; Zhao, T.X.; Huang, M.; Li, H.B. Synthesis, crystal structure and thermal analysis of tetraiodo-4,4’-bi-1,2,4-triazole. Chin. Chem. Lett. 2013, 24, 786–788. [Google Scholar] [CrossRef]
- Il’inykh, E.S.; Kim, D.G.; Kodess, M.I.; Matochkina, E.G.; Slepukhin, P.A. Synthesis of novel fluorine- and iodine-containing [1,2,4]triazolo[3, 4-b][1,3]thiazines based 3-(alkenylthio)-5-(trifluoromethyl)-4H-1,2,4-triazole-3-thiols. J. Fluorine Chem. 2013, 149, 24–29. [Google Scholar] [CrossRef]
- Song, J.; Wu, Z.H.; Wangtrakuldee, B.; Choi, S.R.; Zha, Z.H.; Ploessl, K.; Mach, R.H.; Kung, H. 4-(((4-lodophenyl)methyl)-4H-1,2,4-triazol-4-ylamino)-benzonitrile: A Potential Imaging Agent for Aromatase. J. Med. Chem. 2016, 59, 9370–9380. [Google Scholar] [CrossRef]
- Nagaradja, E.; Bentabed-Ababsa, G.; Scalabrini, M.; Chevallier, F.; Philippot, S.; Fontanay, S.; Duval, R.E.; Halauko, Y.S.; Ivashkevich, O.A.; Matulis, V.E.; et al. Deprotometalation-iodolysis and computed CH acidity of 1,2,3-and 1,2,4-triazoles. Application to the synthesis of resveratrol analogues. Bioorg. Med. Chem. 2015, 23, 6355–6363. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Zhao, T.X.; Li, L.; Zhang, L.J.; Liang, D.H.; Li, H.B. Synthesis and X-Ray Crystal Structures of Tetrahalogeno-4,4’-bi-1,2,4-triazoles. Mol. Cryst. Liq. Cryst. 2015, 623, 333–342. [Google Scholar] [CrossRef]
- Al-Salahi, R.; Al-Omar, M.; Marzouk, M.; Ng, S.W. 5-Chloro-2-methylsulfonyl-1,2,4-triazolo[1,5-a]quinazoline. Acta Cryst. E 2012, 68, o1809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, I.; Panini, P.; Khan, S.U.D.; Rana, U.A.; Andleeb, H.; Chopra, D.; Hameed, S.; Simpson, J. Exploiting the Role of Molecular Electrostatic Potential, Deformation Density, Topology, and Energetics in the Characterization of S⋯N and Cl⋯N Supramolecular Motifs in Crystalline Triazolothiadiazoles. Cryst. Growth Des. 2016, 16, 1371–1386. [Google Scholar] [CrossRef]
- Valkonen, J.; Pitkanen, I.; Pajunen, A. Molecular and crystal structure and IR spectrum of 3,5-dibromo-1,2,4-triazole. Acta Chem. Scand. 1985, 39, 711–716. [Google Scholar] [CrossRef]
- Berski, S.; Ciunik, Z.; Drabent, K.; Latajka, Z.; Panek, J. Dominant role of C–Br⋯N halogen bond in molecular self-organization. Crystallographic and quantum-chemical study of Schiff-base-containing triazoles. J. Phys. Chem. B 2004, 108, 12327–12332. [Google Scholar] [CrossRef]
- Gilandoust, M.; Harsha, K.B.; Madan Kumar, S.; Rakesh, K.S.; Lokanath, N.K.; Byrappa, K.; Rangappa, K.S. 5-Bromo-1,2,4-triazolo[1,5-a]pyrimidine. IUCrData 2016, 1, x161944. [Google Scholar] [CrossRef] [Green Version]
- Eccles, K.S.; Morrison, R.E.; Sinha, A.S.; Maguire, A.R.; Lawrence, S.E. Investigating C=S⋯I Halogen Bonding for Cocrystallization with Primary Thioamides. Cryst. Growth Des. 2015, 15, 3442–3451. [Google Scholar] [CrossRef]
- Eliseeva, A.A.; Ivanov, D.M.; Novikov, A.S.; Kukushkin, V.Y. Recognition of the π-hole donor ability of iodopentafluorobenzene—A conventional σ-hole donor for crystal engineering involving halogen bonding. CrystEngComm 2019, 21, 616–628. [Google Scholar] [CrossRef]
- Mochida, T.; Miura, Y.; Shimizu, F. Assembled Structures and Cation-Anion Interactions in Crystals of Alkylimidazolium and Alkyltriazolium Iodides with Ferrocenyl Substituents. Cryst. Growth Des. 2011, 11, 262–268. [Google Scholar] [CrossRef]
- Guino-o, M.A.; Talbot, M.O.; Snits, M.M.; Pham, T.N.; Audi, M.C.; Janzen, D.E. Crystal structures of five 1-alkyl-4-aryl-1,2,4-triazol-1-ium halide salts. Acta Cryst. E 2015, 71, 628–635. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.Y.; Yuan, J.Y. Poly(1-Vinyl-1,2,4-triazolium) Poly(Ionic Liquid)s: Synthesis and the Unique Behavior in Loading Metal Ions. Macromol. Rapid Commun. 2016, 37, 1124–1129. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Twamley, B.; Shreeve, J.M. The first (ferrocenylmethyl)imidazollum and (ferrocenylmethyl)triazolium room temperature ionic liquids. Inorg. Chem. 2004, 43, 3406–3412. [Google Scholar] [CrossRef]
- Darwich, C.; Karaghiosoff, K.; Klapotke, T.M.; Sabate, C.M. Synthesis and characterization of 3,4,5-Triamino-1,2,4-triazolium and 1-Methyl-3,4,5-triamino-1,2,4-triazolium iodides. Z. Anorg. Allg. Chem. 2008, 634, 61–68. [Google Scholar] [CrossRef]
- McCrary, P.D.; Chatel, G.; Alaniz, S.A.; Cojocaru, O.A.; Beasley, P.A.; Flores, L.A.; Kelley, S.P.; Barber, P.S.; Rogers, R.D. Evaluating Ionic Liquids as Hypergolic Fuels: Exploring Reactivity from Molecular Structure. Energy Fuels 2014, 28, 3460–3473. [Google Scholar] [CrossRef] [Green Version]
- Elnajjar, F.O.; Binder, J.F.; Kosnik, S.C.; Macdonald, C.L.B. 1,2,4-Triazol-5-ylidenes versus Imidazol-2-ylidenes for the Stabilization of Phosphorus(I) Cations. Z. Anorg. Allg. Chem. 2016, 642, 1251–1258. [Google Scholar] [CrossRef]
- Talbot, M.O.; Pham, T.N.; Guino-o, M.A.; Guzei, I.A.; Vinokur, A.I.; Young, V.G. Investigation of ligand steric effect on the hydrogen gas produced via a nickel-catalyzed dehydrogenation of ammonia-borane utilizing unsymmetrical triazolylidene ligands. Polyhedron 2016, 114, 415–421. [Google Scholar] [CrossRef]
- Ghazal, B.; Machacek, M.; Shalaby, M.A.; Novakova, V.; Zimcik, P.; Makhseed, S. Phthalocyanines and Tetrapyrazinoporphyrazines with Two Cationic Donuts: High Photodynamic Activity as a Result of Rigid Spatial Arrangement of Peripheral Substituents. J. Med. Chem. 2017, 60, 6060–6076. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.Q.; Ghiviriga, I.; Pillai, G.G.; Jabeen, F.; Arami, J.A.; Zhou, W.F.; Steel, P.J.; Hall, C.D.; Katritzky, A.R. Synthesis, characterization and energetic properties of novel 1-methyl-1,2,4-triazolium N-aryl/N-pyridinyl ylids. Tetrahedron Lett. 2017, 58, 1079–1085. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.R.; Wang, J.; Yu, Y.H.; Hou, G.F.; Zhang, H.X.; Gao, J.S. Two cationic [(CuxIy)x–y]n motif based coordination polymers and their photocatalytic properties. RSC Adv. 2016, 6, 71206–71213. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Wang, Y.L.; Lin, J.X.; Liu, Q.Y.; Lu, Z.H.; Zhang, N.; Jia Jia, W.; Li, L.Q. Syntheses, structures and properties of coordination polymers of cadmium(II) with 4-methyl-1,2,4-triazole-3-thiol ligand. CrystEngComm 2011, 13, 1697–1706. [Google Scholar] [CrossRef]
- Li, B.Y.; Peng, Y.; Li, G.H.; Hua, J.; Yu, Y.; Jin, D.; Shi, Z.; Feng, S.H. Design and Construction of Coordination Polymers by 4-Amino-3,5-bis(n-pyridyl)-1,2,4-triazole (n = 2, 3, 4) Isomers in a Copper(I) Halide System: Diverse Structures Tuned by Isomeric and Anion Effects. Cryst. Growth Des. 2010, 10, 2192–2201. [Google Scholar] [CrossRef]
- Kuttatheyil, A.V.; Handke, M.; Bergmann, J.; Lassig, D.; Lincke, J.; Haase, J.; Bertmer, M.; Krautscheid, H. 113Cd Solid-State NMR for Probing the Coordination Sphere in Metal-Organic Frameworks. Chem. Eur. J. 2015, 21, 1118–1124. [Google Scholar] [CrossRef]
- Wang, X.; Guo, W.; Guo, Y.M. Controllable assemblies of Cd(II) supramolecular coordination complexes based on a versatile tripyridyltriazole ligand and halide/pseduohalide anions. J. Mol. Struct. 2015, 1096, 136–141. [Google Scholar] [CrossRef]
- Yan, J.Z.; Lu, L.P. Syntheses, Crystal Structures and Luminescence Properties of Two Cu4I4 Coordination Polymers Based on 3,5-Dialkyl-1,2,4-triazole. Chinese J. Inorg. Chem. 2017, 33, 1697–1704. [Google Scholar] [CrossRef]
- Tan, T.T.Y.; Schick, S.; Hahn, F.E. Synthesis and Reactivity of IrIII Complexes Bearing C-Metalated Pyrazolato Ligands. Organometallics 2019, 38, 567–574. [Google Scholar] [CrossRef]
- Libnow, S.; Wille, S.; Christiansen, A.; Hein, M.; Reinke, H.; Kockerling, M.; Miethchen, R. Synthesis and reactivity of halogenated 1,2,4-triazole nucleoside analogues with high potential for chemical modifications. Synth.-Stuttg. 2006, 2006, 496–508. [Google Scholar] [CrossRef]
- Novikov, A.S.; Ivanov, D.M.; Bikbaeva, Z.M.; Bokach, N.A.; Kukushkin, V.Y. Noncovalent Interactions Involving lodofluorobenzenes: The Interplay of Halogen Bonding and Weak Ip(O)⋯π-Hole(arene) Interactions. Cryst. Growth Des. 2018, 18, 7641–7654. [Google Scholar] [CrossRef]
- Bikbaeva, Z.M.; Ivanov, D.M.; Novikov, A.S.; Ananyev, I.V.; Bokach, N.A.; Kukushkin, V.Y. Electrophilic-Nucleophilic Dualism of Nickel(II) toward Ni⋯l Noncovalent Interactions: Semicoordination of Iodine Centers via Electron Belt and Halogen Bonding via σ-Hole. Inorg. Chem. 2017, 56, 13562–13578. [Google Scholar] [CrossRef]
- Bikbaeva, Z.M.; Novikov, A.S.; Suslonov, V.V.; Bokach, N.A.; Kukushkin, V.Y. Metal-mediated reactions between dialkylcyanamides and acetamidoxime generate unusual (nitrosoguanidinate)nickel(II) complexes. Dalton Trans. 2017, 46, 10090–10101. [Google Scholar] [CrossRef] [Green Version]
- Andrusenko, E.V.; Kabin, E.V.; Novikov, A.S.; Bokach, N.A.; Starova, G.L.; Kukushkin, V.Y. Metal-mediated generation of triazapentadienate-terminated di- and trinuclear μ2-pyrazolate NiII species and control of their nuclearity. New J. Chem. 2017, 41, 316–325. [Google Scholar] [CrossRef] [Green Version]
- Mikherdov, A.S.; Novikov, A.S.; Kinzhalov, M.A.; Boyarskiy, V.P.; Starova, G.L.; Ivanov, A.Y.; Kukushkin, V.Y. Halides Held by Bifurcated Chalcogen–Hydrogen Bonds. Effect of μ(S,N–H)Cl Contacts on Dimerization of Cl(carbene)PdII Species. Inorg. Chem. 2018, 57, 3420–3433. [Google Scholar] [CrossRef]
- Mikherdov, A.S.; Kinzhalov, M.A.; Novikov, A.S.; Boyarskiy, V.P.; Boyarskaya, I.A.; Avdontceva, M.S.; Kukushkin, V.Y. Ligation-Enhanced π-Hole⋯π Interactions Involving Isocyanides: Effect of π-Hole⋯π Noncovalent Bonding on Conformational Stabilization of Acyclic Diaminocarbene Ligands. Inorg. Chem. 2018, 57, 6722–6733. [Google Scholar] [CrossRef] [PubMed]
- Adonin, S.A.; Bondarenko, M.A.; Novikov, A.S.; Abramov, P.A.; Sokolov, M.; Fedin, V.P. Halogen bonding in the structures of pentaiodobenzoic acid and its salts. CrystEngComm 2019, 21, 6666–6670. [Google Scholar] [CrossRef]
- Afanasenko, A.M.; Novikov, A.S.; Chulkova, T.G.; Grigoriev, Y.M.; Kolesnikov, I.E.; Selivanov, S.I.; Starova, G.L.; Zolotarev, A.A.; Vereshchagin, A.N.; Elinson, M.N. Intermolecular interactions-photophysical properties relationships in phenanthrene-9,10-dicarbonitrile assemblies. J. Mol. Struct. 2020, 1199, 126789. [Google Scholar] [CrossRef]
- Yandanova, E.S.; Ivanov, D.M.; Kuznetsov, M.L.; Starikov, A.G.; Starova, G.L.; Kukushkin, V.Y. Recognition of S⋯CI Chalcogen Bonding in Metal-Bound Alkylthiocyanates. Cryst. Growth Des. 2016, 16, 2979–2987. [Google Scholar] [CrossRef]
- Bulatova, M.; Melekhova, A.A.; Novikov, A.S.; Ivanov, D.M.; Bokach, N.A. Redox reactive (RNC)CuII species stabilized in the solid state via halogen bond with I2. Z. Kristallogr. Cryst. Mater. 2018, 233, 371–377. [Google Scholar] [CrossRef]
- Rozhkov, A.V.; Novikov, A.S.; Ivanov, D.M.; Bolotin, D.S.; Bokach, N.A.; Kukushkin, V.Y. Structure-Directing Weak Interactions with 1,4-Diiodotetrafluorobenzene Convert One-Dimensional Arrays of [MII(acac)2] Species into Three-Dimensional Networks. Cryst. Growth Des. 2018, 18, 3626–3636. [Google Scholar] [CrossRef]
- Kinzhalov, M.A.; Kashina, M.V.; Mikherdov, A.S.; Mozheeva, E.A.; Novikov, A.S.; Smirnov, A.S.; Ivanov, D.M.; Kryukova, M.A.; Ivanov, A.Y.; Smirnov, S.N.; et al. Dramatically Enhanced Solubility of Halide-Containing Organometallic Species in Diiodomethane: The Role of Solvent⋯Complex Halogen Bonding. Angew. Chem. Int. Ed. 2018, 57, 12785–12789. [Google Scholar] [CrossRef]
- Zelenkov, L.E.; Ivanov, D.M.; Avdontceva, M.S.; Novikov, A.S.; Bokach, N.A. Tetrachloromethane as halogen bond donor toward metal-bound halides. Z. Kristallogr. Cryst. Mater. 2019, 234, 9–17. [Google Scholar] [CrossRef]
- Baykov, S.V.; Dabranskaya, U.; Ivanov, D.M.; Novikov, A.S.; Boyarskiy, V.P. Pt/Pd and I/Br Isostructural Exchange Provides Formation of C–I⋯Pd, C–Br⋯Pt, and C–Br⋯Pd Metal-Involving Halogen Bonding. Cryst. Growth Des. 2018, 18, 5973–5980. [Google Scholar] [CrossRef]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Vener, M.V.; Egorova, A.N.; Churakov, A.V.; Tsirelson, V.G. Intermolecular hydrogen bond energies in crystals evaluated using electron density properties: DFT computations with periodic boundary conditions. J. Comput. Chem. 2012, 33, 2303–2309. [Google Scholar] [CrossRef] [PubMed]
- Bartashevich, E.V.; Tsirelson, V.G. Interplay between non-covalent interactions in complexes and crystals with halogen bonds. Russ. Chem. Rev. 2014, 83, 1181–1203. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Elguero, J.; Molins, E. From weak to strong interactions: A comprehensive analysis of the topological and energetic properties of the electron density distribution involving X–H⋯F–Y systems. J. Chem. Phys. 2002, 117, 5529–5542. [Google Scholar] [CrossRef]







| C–I⋯X | d(I⋯X), Å | RIX b | ∠(C–I⋯X),° |
|---|---|---|---|
| C8S–I3S⋯N2 | 2.913 (6) | 0.83 | 175.3 (2) |
| C1S–I1S⋯N3 | 2.883 (7) | 0.82 | 169.3 (2) |
| C4S–I2S⋯F6S | 3.390 (5) | 0.98 | 149.97 (19) |
| C4S–I2S⋯I3S | 3.8529 (8) | 0.97 | 157.68 (18) |
| Comparison a | 3.53 (I⋯N) 3.45 (I⋯F) 3.96 (I⋯I) | 1.00 | 180 |
| C⋯I–C | d(C⋯I), Å | RCI b | ∠(C⋯I–C),° |
|---|---|---|---|
| C9⋯I1S–C1S | 3.528 (8) | 0.96 | 86.6(3) |
| C9S⋯I2S–C4S | 3.686 (8) | 1.00 | 92.9(3) |
| Comparison a | 3.68 | 1.00 | 90 |
| C–H⋯N | d(H⋯N), Å | RHN b | d(C⋯N), Å | ∠(C–I⋯X),° |
|---|---|---|---|---|
| C7–H7A⋯N5 | 2.484 | 0.90 | 3.441 (10) | 168.6 |
| C16–H16B⋯N4 | 2.733 | 0.99 | 3.62 (1) | 153.9 |
| Comparison a | 2.75 | 1.00 | 3.25 | 110.0 |
| Contact | ρ(r) | ∇2ρ(r) | Hb | V(r) | G(r) | Einta | Eintb | l |
|---|---|---|---|---|---|---|---|---|
| I3S⋯N2 | 0.022 | 0.070 | 0.000 | −0.017 | 0.017 | 5.3 | 4.6 | 2.913 |
| I1S⋯N3 | 0.024 | 0.072 | 0.000 | −0.019 | 0.018 | 6.0 | 4.8 | 2.883 |
| I2S⋯F6S | 0.006 | 0.026 | 0.001 | −0.004 | 0.005 | 1.3 | 1.3 | 3.390 |
| I2S⋯I3S | 0.008 | 0.031 | 0.001 | −0.005 | 0.006 | 1.6 | 1.6 | 3.853 |
| C9⋯I1S | 0.009 | 0.029 | 0.001 | −0.005 | 0.006 | 1.6 | 1.6 | 3.528 |
| C9S⋯I2S | 0.006 | 0.023 | 0.001 | −0.003 | 0.004 | 0.9 | 1.1 | 3.686 |
| H7A⋯N5 | 0.009 | 0.033 | 0.001 | −0.006 | 0.007 | 1.9 | 1.9 | 2.484 |
| H16B⋯N4 | 0.005 | 0.021 | 0.001 | −0.003 | 0.004 | 0.9 | 1.1 | 2.733 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kryukova, M.A.; Sapegin, A.V.; Novikov, A.S.; Krasavin, M.; Ivanov, D.M. New Crystal Forms for Biologically Active Compounds. Part 2: Anastrozole as N-Substituted 1,2,4-Triazole in Halogen Bonding and Lp-π Interactions with 1,4-Diiodotetrafluorobenzene. Crystals 2020, 10, 371. https://doi.org/10.3390/cryst10050371
Kryukova MA, Sapegin AV, Novikov AS, Krasavin M, Ivanov DM. New Crystal Forms for Biologically Active Compounds. Part 2: Anastrozole as N-Substituted 1,2,4-Triazole in Halogen Bonding and Lp-π Interactions with 1,4-Diiodotetrafluorobenzene. Crystals. 2020; 10(5):371. https://doi.org/10.3390/cryst10050371
Chicago/Turabian StyleKryukova, Mariya A., Alexander V. Sapegin, Alexander S. Novikov, Mikhail Krasavin, and Daniil M. Ivanov. 2020. "New Crystal Forms for Biologically Active Compounds. Part 2: Anastrozole as N-Substituted 1,2,4-Triazole in Halogen Bonding and Lp-π Interactions with 1,4-Diiodotetrafluorobenzene" Crystals 10, no. 5: 371. https://doi.org/10.3390/cryst10050371