X-ray Induced Hydroxyl Radical Generation by GdYVO4:Eu3+ Nanoparticles in Aqueous Solution: Main Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of GdYVO4:Eu3+ NPs and Their Characterization
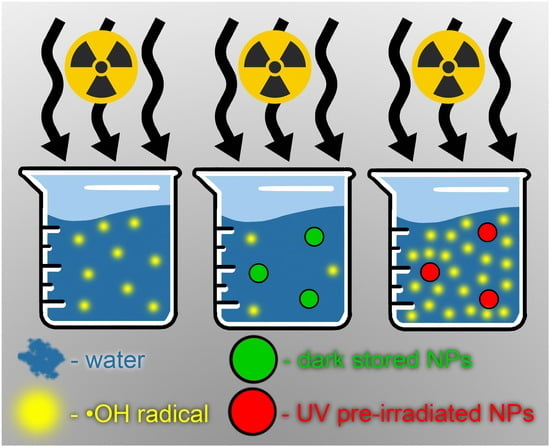
2.2. Measurement of ∙OH Generation in Aqueous Solutions under X-Ray Irradiation
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Delaney, G.; Jacob, S.; Featherstone, C.; Barton, M. The Role of Radiotherapy in Cancer Treatment. Cancer 2005, 104, 1129–1137. [Google Scholar] [CrossRef]
- Grubbé, E.H. Priority in the Therapeutic Use of X-rays. Radiology 1933, 21, 156–162. [Google Scholar] [CrossRef]
- Goel, S.; Ni, D.; Cai, W. Harnessing the Power of Nanotechnology for Enhanced Radiation Therapy. ACS Nano 2017, 11, 5233–5237. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Caer, S. Water radiolysis: Influence of oxide surfaces on H2 Production under ionizing. Radiat. Water 2011, 3, 235–253. [Google Scholar] [CrossRef] [Green Version]
- Buxton, G.V.; Mozumder, A.; Hatano, Y. The Radiation Chemistry of Liquid Water: Principles and Applications. In Charged Particle and Photon Interactions with Matter; Marcel Dekker: New York, NY, USA, 2004; Volume 4, pp. 331–363. [Google Scholar]
- Kwatra, D.; Venugopal, A.; Anant, S. Nanoparticles in Radiation Therapy: A Summary of Various Approaches to Enchance Radiosensitization in Cancer. Transl. Cancer Res. 2016, 2, 330–342. [Google Scholar]
- Haume, K.; Rosa, S.; Grellet, S.; Śmiałek, M.A.; Butterworth, K.T.; Solov’yov, A.V.; Prise, K.M.; Golding, J.; Mason, N.J. Gold nanoparticles for cancer radiotherapy: A review. Cancer Nano 2016, 7, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Wakefield, G.; Gardener, M.; Stock, M.; Adair, M. Nanoparticle Augmented Radiotherapy using Titanium Oxide Nanoparticles. J. Nanomater. Mol. Nanotechnol. 2018, 7, S6:002. [Google Scholar] [CrossRef]
- Brunner, B.; Nestle, U.; Grosu, A.-L.; Partridge, M. SBRT in pancreatic cancer: What is the therapeutic window? Radiother. Oncol. 2014, 114, 109–116. [Google Scholar] [CrossRef]
- Chen, X.; Song, J.; Chen, X.; Yang, H. X-ray-activated nanosystems for theranostic applications. Chem. Soc. Rev. 2019, 48, 3073–3101. [Google Scholar] [CrossRef]
- Paro, A.D.; Shanmugam, I.; van de Ven, A.L. Nanoparticle-Mediated X-Ray Radiation Enhancement for Cancer Therapy. Methods Mol. Biol. 2017, 1530, 391–401. [Google Scholar]
- Kuncic, Z.; Lacombe, S. Nanoparticle radio-enhancement: Principles, progress and application to cancer treatment. Phys. Med. Biol. 2018, 63, 02TR01. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Usami, N.; Porcel, E.; Lacombe, S.; Le Sech, C. Enhancement of radiation effect by heavy elements. Mutat. Res. 2010, 704, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Her, S.; Jaffray, D.A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2015, 109, 84–101. [Google Scholar] [CrossRef]
- Retif, P.; Pinel, S.; Toussaint, M.; Frochot, C.; Chouikrat, C.; Bastogne, T.; Barberi-Heyob, M. Nanoparticles for Radiationh Therapy Enhancement: Key parameters. Theranostics 2015, 5, 1030–1044. [Google Scholar] [CrossRef] [Green Version]
- Butterworth, K.T.; McMahon, S.J.; Currell, F.J.; Prise, K.M. Physical basis and biological mechanisms of gold nanoparticle radiosensitization. Nanoscale 2012, 4, 4830–4838. [Google Scholar] [CrossRef] [PubMed]
- Marill, J.; Anesary, N.M.; Zhang, P.; Vivet, S.; Borghi, E. Hafnium Oxide Nanoparticles: Toward an in vitro predictive biological effect? Radiat. Oncol. 2014, 9, 150–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verry, C.; Sancey, L.; Dufort, S.; Le Duc, G.; Mendoza, C.; Lux, F.; Grand, S.; Arnaud, J.; Quesada, J.L.; Villa, J.; et al. Treatment of multiple brain metastases using gadolinium nanoparticles and radiotherapy: NANO-RAD, a phase I study protocol. BMJ Open 2019, 9, e023591. [Google Scholar] [CrossRef]
- Lux, F.; Sancey, L.; Bianchi, A.; Cremillieux, Y.; Roux, S.; Tillement, O. Gadolinium-based nanoparticles for theranostic MRI-radiosensitization. Nanomedicine 2015, 10, 1801–1816. [Google Scholar] [CrossRef] [Green Version]
- Sancey, L.; Lux, F.; Kot, S.; Roux, S.; Dufort, S.; Bianchi, A. The use of theranostic gadolinium-based nanoprobes to improve radiotherapy efficacy. Br. J. Radiol. 2014, 87, 20140134. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Ridwan, S.M.; Stanishevskiy, Y.; Panchal, R.; Slatkin, D.N.; Smilowitz, H.M. Iodine nanoparticles enhance radiotherapy of intracerebral human glioma in mice and increase efficacy of chemotherapy. Sci. Rep. 2019, 9, 4505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yefimova, S.L.; Maksimchuk, P.O.; Seminko, V.V.; Kavok, N.S.; Klochkov, V.K.; Hubenko, K.A.; Sorokin, A.V.; Kurilchenko, I.Y.; Malyukin, Y.V. Janus-Faced Redox Activity of LnVO4:Eu3+ (Ln = Gd, Y, and La) Nanoparticles. J. Phys. Chem. C 2019, 123, 15323–15329. [Google Scholar] [CrossRef]
- Grygorova, G.; Klochkov, V.; Mamotyuk, Y.; Malyukin, Y. Cerium Dioxide CeO2−x and Orthovanadate (Gd0.9Eu0.1VO4) Nanoparticles for Protection of Living Body from X-Ray Induced Damage. In Nanomaterials for Security; Bonca, J., Kruchinin, S., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 289–296. [Google Scholar]
- Maksimchuk, P.O.; Yefimova, S.L.; Hubenko, K.O.; Omielaieva, V.V.; Kavok, N.S.; Klochkov, V.K.; Sorokin, A.V.; Malyukin, Y.V. Dark Reactive Oxygen Species (ROS) Generation in ReVO4:Eu3+ (Re=Gd, Y) Nanoparticles in Aqueous Solutions. J. Phys. Chem. C 2020, 124, 3843–3850. [Google Scholar] [CrossRef]
- Cooper, D.R.; Bekah, D.; Nadeau, J.L. Gold nanoparticles and their alternatives for radiation therapy enhancement. Front. Chem. 2014, 2, 86. [Google Scholar] [CrossRef] [Green Version]
- Klochkov, V.K. The water solution of nanoluminophores nReVO4:Eu3+ (Re = Y, Gd La). Nanostruct. Materialoved. 2009, 2, 3–8. [Google Scholar]
- Ashawa, S.C.; Kini, U.R.; Madhvanath, U. The Aqueous Coumarin System as a Low Range Chemical Dosimeter. Int. J. Appl. Radiat. Isot. 1979, 30, 7–10. [Google Scholar] [CrossRef]
- Xu, Z.H.; Li, C.X.; Hou, Z.Y.; Pang, C.; Lin, J. Morphological Control and Luminescence Properties of Lanthanide Orthovanadate LnVO4 (Ln= La to Lu) Nano-/microcrystals via Hydrothermal Process. CrystEngComm 2011, 13, 474–482. [Google Scholar] [CrossRef]
- Yang, P.; Huang, S.; Kong, D.; Lin, J.; Fu, H. Luminescence Functionalization of SBA-15 by YVO4: Eu3+ as a Novel Drug Delivery System. Inorg. Chem. 2007, 46, 3203–3211. [Google Scholar] [CrossRef]
- Su, Y.; Li, G.; Xue, Y.; Li, L. Tunable physical properties of CaWO4 nanocrystals via particle size control. J. Phys. Chem. C 2007, 111, 6684–6689. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009; p. 432. [Google Scholar]
- Hsu, C.; Powell, R.C. Energy transfer in Europium doped Yttrium Vanadate crystals. J. Lumin. 1975, 10, 273–293. [Google Scholar] [CrossRef]
- Ronde, H.; Blasse, G. The nature of the electronic transitions of the vanadate group. J. Inorg. Nucl. Chem. 1978, 40, 215–219. [Google Scholar] [CrossRef]
- Yang, L.; Li, G.; Hu, W.; Zhao, M.; Sun, L.; Zheng, J.; Yan, T.; Li, L. Control Over the Crystallinity and Defect Chemistry of YVO4 Nanocrystals for Optimum Photocatalytic Property. Eur. J. Inorg. Chem. 2011, 14, 2211–2220. [Google Scholar] [CrossRef]
- Takeshita, S.; Watanabe, T.; Isobe, T.; Sawayama, T.; Niikura, S. Improvement of the photostability for YVO4:Bi3+,Eu3+ nanoparticles synthesized by the citrate route. Opt. Mater. 2011, 33, 323–326. [Google Scholar] [CrossRef]
- Takeshita, S.; Ogata, H.; Isobe, T.; Sawayama, T.; Niikura, S. Effects of Citrate Additive on Transparency and Photostability Properties of YVO4:Bi3+, Eu3+ Nanophosphor. J. Electrochem. Soc. 2010, 157, 74–80. [Google Scholar] [CrossRef]
- Garces, N.Y.; Stevens, K.T.; Foundos, G.K.; Halliburton, L.E. Electron paramagnetic resonance and optical absorption study of V4+ centers in YVO4 crystals. J. Phys. Condens. Matter. 2004, 16, 7095–7106. [Google Scholar] [CrossRef]
- Lifshitz, I.M. The energy spectrum of disordered systems. Adv. Phys. 1964, 13, 483–536. [Google Scholar] [CrossRef]
- Yefimova, S.L.; Maksimchuk, P.O.; Hubenko, K.A.; Klochkov, V.K.; Borovoy, I.A.; Sorokin, A.V.; Malyukin, Y.V. Untangling the Mechanisms of GdYVO4:Eu3+ Nanoparticle Photocatalytic Activity. Colloid Surf. A 2019, 577, 630–636. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maksimchuk, P.O.; Yefimova, S.L.; Omielaieva, V.V.; Hubenko, K.O.; Klochkov, V.K.; Opolonin, O.D.; Malyukin, Y.V. X-ray Induced Hydroxyl Radical Generation by GdYVO4:Eu3+ Nanoparticles in Aqueous Solution: Main Mechanisms. Crystals 2020, 10, 370. https://doi.org/10.3390/cryst10050370
Maksimchuk PO, Yefimova SL, Omielaieva VV, Hubenko KO, Klochkov VK, Opolonin OD, Malyukin YV. X-ray Induced Hydroxyl Radical Generation by GdYVO4:Eu3+ Nanoparticles in Aqueous Solution: Main Mechanisms. Crystals. 2020; 10(5):370. https://doi.org/10.3390/cryst10050370
Chicago/Turabian StyleMaksimchuk, Pavel O., Svetlana L. Yefimova, Valeriia V. Omielaieva, Kateryna O. Hubenko, Vladimir K. Klochkov, Oleksandr D. Opolonin, and Yuri V. Malyukin. 2020. "X-ray Induced Hydroxyl Radical Generation by GdYVO4:Eu3+ Nanoparticles in Aqueous Solution: Main Mechanisms" Crystals 10, no. 5: 370. https://doi.org/10.3390/cryst10050370