Abstract
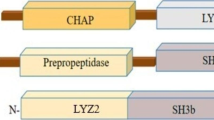
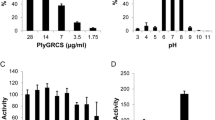
Staphylococcus aureus is one of the most dreadful infectious agents, responsible for high mortality and morbidity in both humans and animals. The increased prevalence of multidrug-resistant (MDR) Staphylococcus aureus strains has limited the number of available treatment options, which calls for the development of alternative and effective modalities against MDR S. aureus. Endolysins are bacteriophage-derived antibacterials, which attack essential conserved elements of peptidoglycan that are vital for bacterial survival, making them promising alternatives or complements to existing antibiotics for tackling such infections. For developing endolysin lysin-methicillin-resistant-5 (LysMR-5) as an effective antimicrobial agent, we evaluated its physical and chemical characteristics, and its intrinsic antibacterial activity against staphylococcal strains, including methicillin-resistant Staphylococcus aureus (MRSA). In this study, we cloned, expressed, and purified LysMR-5 from S. aureus phage MR-5. In silico analysis revealed that LysMR-5 harbors two catalytic and one cell wall-binding domain. Biochemical characterization and LC–MS analysis showed that both catalytic domains were active and had no dependence on divalent ions for their action, Zn2+ exerted a negative effect. The optimal lytic activity of the endolysin was at 37 °C/pH 7.0 and in the presence of ≥ 300 mM concentration of NaCl. Circular dichroism (CD) demonstrated a loss in secondary structure with an increase in temperature confirming the thermosensitive nature of endolysin. Antibacterial assays revealed that LysMR-5 was active against diverse clinical isolates of staphylococci. It showed high lytic efficacy against S. aureus ATCC 43300, as an endolysin concentration as low as 15 µg/ml was sufficient to achieve maximum lytic activity within 30 min and it was further confirmed by scanning electron microscopy. Our results indicate that rapid and strong bactericidal activity of LysMR-5 makes it a valuable candidate for eradicating multidrug-resistant S. aureus.









Similar content being viewed by others
References
Lowy FD (1998) Staphylococcus aureus infections. N Engl J Med 339:520–532
Ferry T, Perpoint T, Vandenesch F, Etienne J (2005) Virulence determinants in Staphylococcus aureus and their involvement in clinical syndromes. Curr Infect Dis Rep 7:420–428
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281
Joshi S, Ray P, Manchanda V, Bajaj J, Chitnis DS, Gautam V, Goswami P, Gupta V, Harish BN, Kagal A, Kapil A, Rao R, Rodrigues C, Sardana R, Devi S, Sharma A (2013) Veeragaghavan Balaji Indian Network for Surveillance of Antimicrobial Resistance (INSAR) group, India. Methicillin resistant Staphylococcus aureus (MRSA) in India: prevalence & susceptibility pattern. Indian J Med Res 137:363–369
Boswihi SS, Udo EE (2018) Methicillin-resistant Staphylococcus aureus: An update on the epidemiology, treatment options and infection control. Curr Med Res Pract 8:18–24
Wu M, Hu K, Xie Y, Liu Y, Mu D, Guo H, Zhang Z, Zhang Y, Chang D, Shi Y (2019) A Novel phage PD-6A3, and its endolysin Ply6A3, with extended lytic activity against Acinetobacter baumannii. Front Microbiol. https://doi.org/10.3389/fmicb.2018.03302
Donovan DM, Becker SC, Dong S, Baker JR, Foster-Frey J, Pritchard DG (2009) Peptidoglycan hydrolase enzyme fusions for treating multi-drug resistant pathogens. Biotech Int 21:6–10
Son B, Yun J, Lim JA, Shin H, Heu S, Ryu S (2012) Characterization of LysB4, an endolysin from the Bacillus cereus-infecting bacteriophage B4. BMC Microbiol 12:33
Haddad KH, Schmelcher M, Sabzalipoor H, Seyed HE, Moniri R (2018) Recombinant endolysins as potential therapeutics against antibiotic-resistant Staphylococcus aureus: current status of research and novel delivery strategies. Clin Microbiol Rev 31:e00071–e00117
Melo LDR, Brandao A, Akturk E, Santos SB, Azeredo J (2018) Characterization of a new Staphylococcus aureus kayvirus harboring a lysin active against biofilms. Viruses 10:182. https://doi.org/10.3390/v10040182
Loessner MJ (2005) Bacteriophage endolysins: current state of research and applications. Curr Opin Microbiol 8:480–487
Fischetti VA (2010) Bacteriophage endolysins: a novel anti-infective to control Gram-positive pathogens. Int J Med Microbiol 300:357–362
Oliveira H, São-José C, Azeredo J (2018) Phage-derived peptidoglycan degrading enzymes: challenges and future prospects for in vivo therapy. Viruses 10:E292. https://doi.org/10.3390/v10060292
Loeffler JM, Nelson D, Fischetti VA (2001) Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 294:2170–2172
Fischetti VA (2005) Bacteriophage lytic enzymes: novel anti-infectives. Trends Microbiol 13:491–496
Gutiérrez DFL, Rodríguez A, García P (2018) Are phage lytic proteins the secret weapon to kill Staphylococcus aureus? mBio 9:e01923–e02017
Schmelcher M, Donovan DM, Loessner MJ (2012) Bacteriophage endolysins as novel antimicrobials. Future Microbiol 7:1147–1171
Jun SY, Jung GM, Yoon SJ, Oh MD, Choi YJ, Lee WJ, Kong JC, Seol JG, Kang SH (2013) Antibacterial properties of a pre-formulated recombinant phage endolysin, SAL-1. Int J Antimicrob Agents 41:156–161
O'Flaherty S, Coffey A, Meaney W, Fitzgerald GF, Ross RP (2005) The recombinant phage lysin LysK has a broad spectrum of lytic activity against clinically relevant staphylococci, including methicillin-resistant Staphylococcus aureus. J Bacteriol 187:7161–7164
Rashel M, Uchiyama J, Ujihara T, Uehara Y, Kuramoto S, Sugihara S, Yagyu K, Muraoka A, Sugai M, Hiramatsu K, Honke K, Matsuzaki S (2007) Efficient elimination of multidrug-resistant Staphylococcus aureus by cloned lysin derived from bacteriophage phi MR11. J Infect Dis 196:1237–1247
Drulis-Kawa Z, Majkowska-Skrobek G, Maciejewska B (2015) Bacteriophages and phage-derived proteins–application approaches. Curr Med Chem 22:1757–1773
Heselpoth RD, Swift SM, Linden SB, Mitchell MS, Nelson DC (2018) Enzybiotics: endolysins and bacteriocins. In: Harper D, Abedon ST, Burrowes B, McConvill M (eds) Bacteriophages. Springer, Cham, pp 1–42
Fernández-Ruiz I, Coutinho FH, Rodriguez-Valera F (2018) Thousands of novel endolysins discovered in uncultured phage genomes. Front Microbiol 9:1033. https://doi.org/10.3389/fmicb.2018.01033
Loeffler JM, Fischetti VA (2003) Synergistic lethal effect of a combination of phage lytic enzymes with different activities on penicillin-sensitive and -resistant Streptococcus pneumoniae strains. Antimicrob Agents Chemother 47:375–377
Gu J, Xu W, Lei L, Huang J, Feng X, Sun C, Du C, Zuo J, Li Y, Du T, Li L, Han W (2010) Lysgh15, a novel bacteriophage lysin, protects a murine bacteremia model efficiently against lethal methicillin-resistant Staphylococcus aureus infection. J Clin Microbiol 49:111–117
Jun SY, Jung GM, Yoon SJ, Choi YJ, Koh WS, Moon KS, Kang SH (2014) Preclinical safety evaluation of intravenously administered SAL200 containing the recombinant phage endolysin SAL-1 as a pharmaceutical ingredient. Antimicrob Agents Chemother 58:2084–2088. https://doi.org/10.1128/AAC.02232-13
Loessner MJ, Gaeng S, Wendlinger G, Maier SK, Scherer S (1998) The two-component lysis system of Staphylococcus aureus bacteriophage Twort: a large TTG-start holin and an associated amidase endolysin. FEMS Microbiol Lett 162:265–274
Sass P, Bierbaum G (2007) Lytic activity of recombinant bacteriophage phi11 and phi12 endolysins on whole cells and biofilms of Staphylococcus aureus. Appl Environ Microbiol 73:347–352
Daniel A, Euler C, Collin M, Chahales P, Gorelick KJ, Fischetti VA (2010) Synergism between a novel chimeric lysin and oxacillin protects against infection by methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 54:1603–1612
Kaur S, Harjai K, Chhibber S (2012) Methicillin-resistant Staphylococcus aureus phage plaque size enhancement using sublethal concentrations of antibiotics. Appl Environ Microbiol 78:8227–8233
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor, New York
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor, New York
He F (1997) Laemmli-SDSPAGE Bio-protocol. Bio101. https://doi.org/10.21769/BioProtoc.80
Corpet F (1988) Multiple sequence alignment with hierarchical clustering. Nucl Acids Res 16:10881–10890
Geourjon C, Deleage G (1995) SOPMA: significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Comput Appl Biosci 11:681–684. https://doi.org/10.1093/bioinformatics/11.6.681
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714–2723. https://doi.org/10.1002/elps.1150181505
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. https://doi.org/10.1002/jcc.20084
Binkowski TA, Naghibzadeh S, Liang J (2003) CASTp: Computed Atlas of Surface Topography of proteins. Nucleic Acids Res 31:3352–3355. https://doi.org/10.1093/nar/gkg512
Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26:283–291. https://doi.org/10.1107/S0021889892009944
Leive L (1968) Studies on the permeability change produced in coliform bacteria by ethylenediaminetetraacetate. J Biol Chem 243:2373–2380
Lepeuple AS, Gemert EV, Chapot-Chartier MP (1998) Analysis of the bacteriolytic enzymes of the autolytic Lactococcus lactis subsp. cremoris strain AM2 by renaturing polyacrylamide gel electrophoresis: identification of a prophage-encoded enzyme. Appl Environ Microbiol 64:4142–4148
Lavigne R, Briers Y, Hertveldt K, Robben J, Volckaert G (2004) Identification and characterization of a highly thermostable bacteriophage lysozyme. Cell Mol Life Sci 61:2753–2759
Pritchard DG, Dong S, Kirk MC, Cartee RT, Baker JR (2007) LambdaSa1 and LambdaSa2 prophage lysins of Streptococcus agalactiae. Appl Environ Microbiol 73:7150–7154
Mokrasch LC (1967) Use of 2,4,6-trinitrobenzenesulfonic acid for the coestimation of amines, amino acids, and proteins in mixtures. Anal Biochem 18:64–71
Hazenberg MP, de Visser H (1992) Assay for N-acetylmuramyl-l-alanine amidase in serum by determination of muramic acid released from the peptidoglycan of Brevibacterium divaricatum. Eur J Clin Chem Clin Biochem 30:141–144
Park JT, Johnson MJ (1949) A submicrodetermination of glucose. J Biol Chem 181:149–151
Becker SC, Dong S, Baker JR, Foster-Frey J, Pritchard DG, Donovan DM (2009) LysK CHAP endopeptidase domain is required for lysis of live staphylococcal cells. FEMS Microbiol Lett 294:52–60
Briers Y, Lavigne R, Volckaert G, Hertveldt K (2007) A standardized approach for accurate quantification of murein hydrolase activity in high-throughput assays. J Biochem Biophys Methods 70:531–533
Nelson D, Loomis L, Fischetti VA (2001) Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc Natl Acad Sci USA 98:4107–4112
Fenton M, Cooney JC, Ross RP, Sleator RD, McAuliffe O, O’Mahony J, Coffey A (2011) In silico modeling of the staphylococcal bacteriophage-derived peptidase CHAPK. Bacteriophage 1:198–206
Gu J, Feng Y, Feng X, Sun C, Lei L, Ding W, Niu F, Jiao L, Yang M, Li Y, Liu X, Song J, Cui Z, Han D, Du C, Yang Y, Ouyang S, Liu ZJ, Han W (2014) Structural and biochemical characterization reveals LysGH15 as an unprecedented “EF-Hand-Like” calcium-binding phage lysin. PLOS Pathog 10:e1004109
Fujiki J, Nakamura T, Furusawa T, Ohno H, Takahashi H, Kitana J, Usui M, Higuchi H, Tanji Y, Tamura Y, Iwano H (2018) Characterization of the lytic capability of a LysK-like endolysin, Lys-phiSA012, derived from a polyvalent Staphylococcus aureus bacteriophage. Pharmaceuticals (Basel) 11:E25. https://doi.org/10.3390/ph11010025
Fischetti VA (2008) Bacteriophage lysins as effective antibacterials. Curr Opin Microbiol 11:393–400
Fenton M, Casey PG, Hill C, Gahan CG, Ross RP, McAuliffe O, O'Mahony J, Maher F, Coffey A (2010) The truncated phage lysin CHAP(k) eliminates Staphylococcus aureus in the nares of mice. Bioeng Bugs 1:404–407
Singh PK, Donovan DM, Kumar A (2014) Intravitreal injection of the chimeric phage endolysin Ply187 protects mice from Staphylococcus aureus endophthalmitis. Antimicrob Agents Chemother 58:4621–4629
Oliveira H, Melo LD, Santos SB, Nóbrega FL, Ferreira EC, Cerca N, Azeredo J, Kluskens LD (2013) Molecular aspects and comparative genomics of bacteriophage endolysins. J Virol 87:4558–4570
Navarre WW, Schneewind O (1999) Surface proteins of Gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol Mol Biol Rev 63:174–229
Díaz E, López R, García JL (1990) Chimeric phage-bacterial enzymes: a clue to the modular evolution of genes. Proc Natl Acad Sci USA 87:8125–8129
Donovan DM, Lardeo M, Foster-Frey J (2006) Lysis of staphylococcal mastitis pathogens by bacteriophage phi11 endolysin. FEMS Microbiol Lett 265:133–139
Horgan M, O’Flynn G, Garry J, Cooney J, Coffey A, Fitzgerald GF, Ross RP, McAuliffe O (2009) Phage lysin LysK can be truncated to its CHAP domain and retain lytic activity against live antibiotic-resistant staphylococci. Appl Environ Microbiol 75:872–874
Rodríguez-Rubio L, Martínez B, Rodríguez A, Donovan DM, Götz F, García P (2013) The phage lytic proteins from the Staphylococcus aureus bacteriophage vB_SauS-phiIPLA88 display multiple active catalytic domains and do not trigger staphylococcal resistance. PLoS ONE 8:e64671. https://doi.org/10.1371/journal.pone.0064671
Schmelcher M, Shen Y, Nelson DC, Eugster MR, Eichenseher F, Hanke DC, Loessner MJ, Dong S, Pritchard DG, Lee JC, Becker SC, Foster-Frey J, Donovan DM (2015) Evolutionarily distinct bacteriophage endolysins featuring conserved peptidoglycan cleavage sites protect mice from MRSA infection. J Antimicrob Chemother 70:1453–1465
Rodríguez-Rubio L, Martínez B, Zhou Y, Rodríguez A, Donovan DM, García P (2011) Lytic activity of the virion-associated peptidoglycan hydrolase HydH5 of Staphylococcus aureus bacteriophage vB_SauS-phiIPLA88. BMC Microbiol 11:138
Jun SY, Jung GM, Son JS, Yoon SJ, Choi YJ, Kang SH (2011) Comparison of the antibacterial properties of phage endolysins SAL-1 and LysK. Antimicrob Agents Chemother 55:1764–1767
Celia LK, Nelson D, Kerr DE (2008) Characterization of a bacteriophage lysin (Ply700) from Streptococcus uberis. Vet Microbiol 130:107–117
Pritchard DG, Dong S, Baker JR, Engler JA (2004) The bifunctional peptidoglycan lysin of Streptococcus agalactiae bacteriophage B30. Microbiology 150:2079–2087
Becker SC, Foster-Frey J, Donovan DM (2008) The phage K lytic enzyme LysK and lysostaphin act synergistically to kill MRSA. FEMS Microbiol Lett 287:185–191
Filatov LY, Becker SC, Donovan DM, Gladilin AK, Klyachko NL (2010) Lysk, the enzyme lysing Staphylococcus aureus cells: specific kinetic features and approaches towards stabilization. Biochimie 92:507–513
Oliveira H, Thiagarajan V, Walmagh M, Sillankorva S, Lavigne R, Neves-Petersen MT, Kluskens LD, Azeredo J (2014) A thermostable Salmonella phage endolysin, Lys68, with broad bactericidal properties against gram-negative pathogens in presence of weak acids. PLoS ONE 9:e108376. https://doi.org/10.1371/journal.pone.0108376
Oliveira H, Vilas BD, Mesnage S, Kluskens LD, Lavigne R, Sillankorva S, Secundo F, Azeredo J (2016) Structural and enzymatic characterization of ABgp46, a novel phage endolysin with broad anti-gram-negative bacterial activity. Front Microbiol 7:208. https://doi.org/10.3389/fmicb.2016.00208
Cheng X, Zhang X, Pflugrath JW, Studier FW (1994) The structure of bacteriophage T7 lysozyme, a zinc amidase and an inhibitor of T7 RNA polymerase. Proc Natl Acad Sci USA 9:4034–4038
Park J, Yun J, Lim JA, Kang DH, Ryu S (2012) Characterization of an endolysin, LysBPS13, from a Bacillus cereus bacteriophage. FEMS Microbiol Lett 33:76–83
Cano IG, Campos-Gómez M, Contreras-Cruz M et al (2015) Expression, purification, and characterization of a bifunctional 99-kDa peptidoglycan hydrolase from Pediococcus acidilactici ATCC 8042. Appl Microbiol Biotechnol 99:8563–8573
Becker SC, Swift S, Korobova O, Schischkova N, Kopylov P, Donovan DM, Abaev I (2015) Lytic activity of the staphylolytic Twort phage endolysin CHAP domain is enhanced by the SH3b cell wall binding domain. FEMS Microbiol Lett 362:1–8
Uchiyama J, Takemura I, Hayashi I, Matsuzaki S, Satoh M, Ujihara T, Murakami M, Imajoh M, Sugai M, Daibata M (2011) Characterization of lytic enzyme open reading frame 9 (ORF9) derived from Enterococcus faecalis bacteriophage phiEF24C. Appl Environ Microbiol 77:580–585
Plotka M, Kaczorowska AK, Morzywolek A, Makowska J, Kozlowski LP, Thorisdottir A, Skírnisdottir S, Hjörleifsdottir S, Fridjonsson OH, Hreggvidsson GO, Kristjansson JK, Dabrowski S, Bujnicki JM, Kaczorowski T (2015) Biochemical characterization and validation of a catalytic site of a highly thermostable Ts2631 endolysin from the Thermus scotoductus Phage vB_Tsc2631. PLoS ONE 10:e0137374
Plotka M, Sancho-Vaello E, Dorawa S, Kaczorowska AK, Kozlowski LP, KaczorowskI T, Zeth K (2019) Structure and function of the Ts2631 endolysin of Thermus scotoductus phage vB_Tsc2631 with unique N-terminal extension used for peptidoglycan binding. Sci Rep. https://doi.org/10.1038/s41598-018-37417-6
Guo M, Feng C, Ren J, Zhuang X, Zhang Y, Zhu Y, Dong K, He P, Guo X, Qin J (2017) A novel antimicrobial endolysin, LysPA26, against Pseudomonas aeruginosa. Front Microbiol 8:293
Lai MJ, Lin NT, Hu A, Soo PC, Chen LK, Chen LH, Chang KC (2011) Antibacterial activity of Acinetobacter baumannii phage ϕAB2 endolysin (LysAB2) against both gram-positive and gram-negative bacteria. Appl Microbiol Biotechnol 90:529–539
Walmagh M, Boczkowska B, Grymonprez B, Briers Y, Drulis-Kawa Z, Lavigne R (2013) Characterization of five novel endolysins from Gram-negative infecting bacteriophages. Appl Microbiol Biotechnol 97:4369–4375
Acknowledgments
We gratefully acknowledge the University Grant Commission, New Delhi, India, and DST-PURSE grant, New Delhi for the financial support for the purchase of consumables and Ashutosh Kumar for assistance with the cloning of the endolysin gene in this project.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: S.C., J.K. Performed the experiments: J.K., P.S. Analyzed the data: S.C., J.K., P.S., D.S., K.H. Contributed reagents/materials/analysis tools: S.C., D.S., K.H. Wrote the paper: S.C., J.K., P.S., D.S., KH.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual authors included in this article.
Additional information
Edited by Andrew Millard.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kaur, J., Singh, P., Sharma, D. et al. A potent enzybiotic against methicillin-resistant Staphylococcus aureus. Virus Genes 56, 480–497 (2020). https://doi.org/10.1007/s11262-020-01762-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-020-01762-4