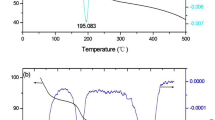
Abstract
Good dispersibility of graphene in a medium or matrix is a critical issue in practical applications. In this work, graphene was functionalized using N-(4-hydroxyl phenyl) maleimide (4-HPM) via the Diels—Alder (DA) reaction by a one-step catalyst-free approach. The optimal reaction condition was found to be 90 °C for 12 h using dimethylformamide (DMF) as the solvent. FTIR, Raman spectroscopy, XPS and EDS proved that 4-HPM moieties were successfully grafted onto the surface of graphene. UV-vis and TGA confirmed that the grafting amount of 4-HPM was 3.75%–3.97% based on the mass of graphene. Functionalized graphene showed excellent dispersion stability when dispersed in common solvents such as ethanol, DMF, water, tetrahydrofuran and p-xylene. Meanwhile, functionalized graphene also exhibited pH sensitivity in aqueous due to the phenolic hydroxyls from the 4-HPM moieties. As a result of good dispersion stability and pH sensitivity, compared with graphene, functionalized graphene had better adsorption capacity for methylene blue (MB) from aqueous solution.
Similar content being viewed by others
References
Novoselov K S, Geim A K, Morozov S V, et al. Electric field effect in atomically thin carbon films. Science, 2004, 306(5696): 666–669
Du X, Skachko I, Barker A, et al. Approaching ballistic transport in suspended graphene. Nature Nanotechnology, 2008, 3(8): 491–495
Nair R R, Blake P, Grigorenko A N, et al. Fine structure constant defines visual transparency of graphene. Science, 2008, 320(5881): 1308
Lee C, Wei X, Kysar J W, et al. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science, 2008, 321(5887): 385–388
Balandin A A, Ghosh S, Bao W, et al. Superior thermal conductivity of single-layer graphene. Nano Letters, 2008, 8(3): 902–907
Wu J B, Lin M L, Cong X, et al. Raman spectroscopy of graphene-based materials and its applications in related devices. Chemical Society Reviews, 2018, 47(5): 1822–1873
Kuila T, Mishra A K, Khanra P, et al. Recent advances in the efficient reduction of graphene oxide and its application as energy storage electrode materials. Nanoscale, 2013, 5(1): 52–71
Zhu C, Liu T, Qian F, et al. Supercapacitors based on three-dimensional hierarchical graphene aerogels with periodic macropores. Nano Letters, 2016, 16(6): 3448–3456
Brownson D A C, Kampouris D K, Banks C E. An overview of graphene in energy production and storage applications. Journal of Power Sources, 2011, 196(11): 4873–4885
Bai S, Shen X. Graphene-inorganic nanocomposites. RSC Advances, 2012, 2(1): 64–98
Huang X, Qi X, Boey F, et al. Graphene-based composites. Chemical Society Reviews, 2012, 41(2): 666–686
Liang Y, Wang H, Zhou J, et al. Covalent hybrid of spinel manganese-cobalt oxide and graphene as advanced oxygen reduction electrocatalysts. Journal of the American Chemical Society, 2012, 134(7): 3517–3523
Xiang Q, Yu J, Jaroniec M. Graphene-based semiconductor photocatalysts. Chemical Society Reviews, 2012, 41(2): 782–796
Lee H, Choi T K, Lee Y B, et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nature Nanotechnology, 2016, 11(6): 566–572
Qiu X, Liu X, Zhang W, et al. Dynamic monitoring of microRNA-DNA hybridization using DNAase-triggered signal amplification. Analytical Chemistry, 2015, 87(12): 6303–6310
Yan L, Zheng Y B, Zhao F, et al. Chemistry and physics of a single atomic layer: strategies and challenges for functionalization of graphene and graphene-based materials. Chemical Society Reviews, 2012, 41(1): 97–114
Xu Y, Zhao L, Bai H, et al. Chemically converted graphene induced molecular flattening of 5,10,15,20-tetrakis(1-methyl-4-pyridinio)porphyrin and its application for optical detection of cadmium(II) ions. Journal of the American Chemical Society, 2009, 131(37): 13490–13497
Parviz D, Das S, Ahmed H S T, et al. Dispersions of noncovalently functionalized graphene with minimal stabilizer. ACS Nano, 2012, 6(10): 8857–8867
Park S, An J, Piner R D, et al. Aqueous suspension and characterization of chemically modified graphene sheets. Chemistry of Materials, 2008, 20(21): 6592–6594
Ayán-Varela M, Paredes J I, Guardia L, et al. Achieving extremely concentrated aqueous dispersions of graphene flakes and catalytically efficient graphene-metal nanoparticle hybrids with flavin mononucleotide as a high-performance stabilizer. ACS Applied Materials & Interfaces, 2015, 7(19): 10293–10307
Pei S, Wei Q, Huang K, et al. Green synthesis of graphene oxide by seconds timescale water electrolytic oxidation. Nature Communications, 2018, 9(1): 145
Hummers W S, Offeman R E. Preparation of graphitic oxide. Journal of the American Chemical Society, 1958, 80(6): 1339
Robinson J T, Burgess J S, Junkermeier C E, et al. Properties of fluorinated graphene films. Nano Letters, 2010, 10(8): 3001–3005
Nair R R, Ren W, Jalil R, et al. Fluorographene: a two-dimensional counterpart of Teflon. Small, 2010, 6(24): 2877–2884
Lundstedt A, Papadakis R, Li H, et al. White-light photoassisted covalent functionalization of graphene using 2-propanol. Small Methods, 2017, 1(11): 1700214
Lomeda J R, Doyle C D, Kosynkin D V, et al. Diazonium functionalization of surfactant-wrapped chemically converted graphene sheets. Journal of the American Chemical Society, 2008, 130(48): 16201–16206
Konios D, Stylianakis M M, Stratakis E, et al. Dispersion behaviour of graphene oxide and reduced graphene oxide. Journal of Colloid and Interface Science, 2014, 430: 108–112
Cao Y, Lai Z, Feng J, et al. Graphene oxide sheets covalently functionalized with block copolymers via click chemistry as reinforcing fillers. Journal of Materials Chemistry, 2011, 21(25): 9271–9278
Beck M T, Szépvölgyi J, Szabó P, et al. Heterogeneous Diels—Alder reaction between cyclopentadiene and different solid carbons. Carbon, 2001, 39(1): 147–149
Zhu J, Hiltz J, Mezour M A, et al. Facile covalent modification of a highly ordered pyrolytic graphite surface via an inverse electron demand Diels—Alder reaction under ambient conditions. Chemistry of Materials, 2014, 26(17): 5058–5062
Zydziak N, Hübner C, Bruns M, et al. One-step functionalization of single-walled carbon nanotubes (SWCNTs) with cyclopenta-dienyl-capped macromolecules via Diels—Alder chemistry. Macromolecules, 2011, 44(9): 3374–3380
Le C M Q, Cao X T, Lim K T. Ultrasound-promoted direct functionalization of multi-walled carbon nanotubes in water via Diels—Alder “click chemistry”. Ultrasonics Sonochemistry, 2017, 39: 321–329
Sarkar S, Bekyarova E, Haddon R C. Chemistry at the Dirac point: Diels—Alder reactivity of graphene. Accounts of Chemical Research, 2012, 45(4): 673–682
Cao Y, Osuna S, Liang Y, et al. Diels—Alder reactions of graphene: computational predictions of products and sites of reaction. Journal of the American Chemical Society, 2013, 135(46): 17643–17649
Denis P A. Diels—Alder reactions onto fluorinated and hydrogenated graphene. Chemical Physics Letters, 2017, 684: 79–85
Yuan J, Chen G, Weng W, et al. One-step functionalization of graphene with cyclopentadienyl-capped macromolecules via Diels—Alder “click” chemistry. Journal of Materials Chemistry, 2012, 22(16): 7929–7936
Liu J, Zeng X, Zhang X, et al. Synthesis and curing properties of a novel curing agent based on N-(4-hydroxyphenyl) maleimide and dicyclopentadiene moieties. Journal of Applied Polymer Science, 2011, 120(1): 56–61
Sarkar S, Bekyarova E, Niyogi S, et al. Diels—Alder chemistry of graphite and graphene: graphene as diene and dienophile. Journal of the American Chemical Society, 2011, 133(10): 3324–3327
Ferrari A C, Meyer J C, Scardaci V, et al. Raman spectrum of graphene and graphene layers. Physical Review Letters, 2006, 97(18): 187401
Wu J B, Lin M L, Cong X, et al. Raman spectroscopy of graphene-based materials and its applications in related devices. Chemical Society Reviews, 2018, 47(5): 1822–1873
Cledera-Castro M, Santos-Montes A, Izquierdo-Hornillos R, et al. Comparison of the performance of different reversed-phase columns for liquid chromatography separation of 11 pollutant phenols. Journal of Separation Science, 2007, 30(5): 699–707
Zhang Y, Xu Y. Simultaneous electrochemical dual-electrode exfoliation of graphite toward scalable production of high-quality graphene. Advanced Functional Materials, 2019, 29(37): 1902171
Seo J, Jeon I, Baek J. Mechanochemically driven solid-state Diels—Alder reaction of graphite into graphene nanoplatelets. Chemical Science, 2013, 4(11): 4273–4277
Redelius P. Bitumen solubility model using Hansen solubility parameter. Energy & Fuels, 2004, 18(4): 1087–1092
Jang B N, Wang D, Wilkie C A. Relationship between the solubility parameter of polymers and the clay dispersion in polymer/clay nanocomposites and the role of the surfactant. Macromolecules, 2005, 38(15): 6533–6543
Acknowledgement
The authors would like to thank the National Natural Science Foundation of China (Grant No. 51573211) for financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosure of potential conflicts of interest There is no conflict of interest to declare.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhang, J., Hu, K., Ouyang, Q. et al. One-step functionalization of graphene via Diels—Alder reaction for improvement of dispersibility. Front. Mater. Sci. 14, 198–210 (2020). https://doi.org/10.1007/s11706-020-0501-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11706-020-0501-0