Abstract
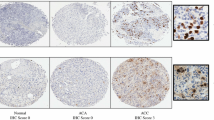
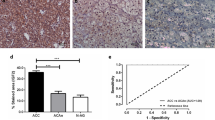
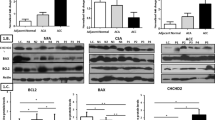
Metabolic reprogramming is a cellular process contributing to carcinogenesis. However, it remains poorly understood in adrenal cortical carcinoma (ACC), an aggressive malignancy with overall poor prognosis and limited therapeutic options. We characterized the metabolic phenotype of ACC, by examining the immunoprofile of key proteins involved in glucose metabolism, hexokinase (HK1), pyruvate kinase (PKM1, PKM2), succinate dehydrogenase (SDHB), and phospho-S6 ribosomal protein (pS6), in a tissue microarray of 137 adrenal cortical tissue samples. Protein expression was compared between ACC (n = 42), adrenal cortical adenoma (ACA; n = 50), and normal adrenal cortical tissue samples (n = 45). Cytoplasmic expression of HK1 and PKM2 was significantly higher in ACC than in ACA (p < 0.001 and p = 0.014, respectively) or normal adrenal cortical tissue samples (p < 0.001 and p < 0.001, respectively). Expression of HK1 and PKM2 was also higher in ACA than in normal adrenal cortical tissue samples (p < 0.001 and p < 0.001, respectively). PKM1 expression was overall low in ACC, ACA, and normal samples, although expression of PKM1 was higher in ACC than in ACA (p = 0.027). There was no loss of cytoplasmic granular SDHB expression in our cohort of adrenal cortical tumors, and cytoplasmic expression of pS6 was lower in ACC than in ACA (p = 0.003) or normal adrenal cortical tissue samples (p = 0.008). Significantly, HK1 expression correlated with pyruvate kinase isoform (PKM2 and PKM1) expression (p < 0.001 and p = 0.007, respectively). Although functional validation was not performed, this study provides further evidence that metabolic reprogramming and altered glucose metabolism may occur in a subset of ACC through overexpression of intracellular glycolytic enzymes, notably HK1 and PKM2. The possibility of utilizing the reprogrammed glucose metabolism in ACC for novel therapeutic strategies should be explored in future studies.



Similar content being viewed by others
References
Duan K, Giordano TJ, Mete O (2016) Adrenal cortical proliferations. In: Mete O, Asa SL, eds. Endocrine Pathology. UK: Cambridge University Press; 602–627.
Zheng S, Cherniack AD, Dewal N, et al (2016) Comprehensive Pan-Genomic Characterization of Adrenocortical Carcinoma. Cancer Cell 29:723–736
Libè R, Fratticci A, Bertherat J (2007) Adrenocortical cancer: pathophysiology and clinical management. Endocr Relat Cancer 14:13–28
Giordano TJ, Kuick R, Else T, Gauger PG, Vinco M, Bauersfeld J, Sanders D, Thomas DG, Doherty G, Hammer G (2009) Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin Cancer Res Off J Am Assoc Cancer Res 15:668–676
de Reyniès A, Assié G, Rickman DS, Tissier F, Groussin L, René-Corail F, Dousset B, Bertagna X, Clauser E, Bertherat J (2009) Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival. J Clin Oncol Off J Am Soc Clin Oncol 27:1108–1115
Assié G, Letouzé E, Fassnacht M, et al (2014) Integrated genomic characterization of adrenocortical carcinoma. Nat Genet 46:607–612
Mete O, Gucer H, Kefeli M, Asa SL (2018) Diagnostic and Prognostic Biomarkers of Adrenal Cortical Carcinoma. Am J Surg Pathol 42:201–213
Pinheiro C, Granja S, Longatto-Filho A, Faria AM, Fragoso MCBV, Lovisolo SM, Lerário AM, Almeida MQ, Baltazar F, Zerbini MCN (2015) Metabolic reprogramming: a new relevant pathway in adult adrenocortical tumors. Oncotarget 6:44403–44421
Pinheiro C, Granja S, Longatto-Filho A, et al (2017) GLUT1 expression in pediatric adrenocortical tumors: a promising candidate to predict clinical behavior. Oncotarget. https://doi.org/10.18632/oncotarget.19135
Fenske W, Völker H-U, Adam P, et al (2009) Glucose transporter GLUT1 expression is an stage-independent predictor of clinical outcome in adrenocortical carcinoma. Endocr Relat Cancer 16:919–928
Liberti MV, Locasale JW (2016) The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci 41:211–218
Vander Heiden MG, DeBerardinis RJ (2017) Understanding the Intersections between Metabolism and Cancer Biology. Cell 168:657–669
Sullivan LB, Gui DY, Heiden MGV (2016) Altered metabolite levels in cancer: implications for tumour biology and cancer therapy. Nat Rev Cancer 16:680–693
Hay N (2016) Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat Rev Cancer 16:635–649
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324:1029–1033
Vander Heiden MG, Locasale JW, Swanson KD, et al (2010) Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science 329:1492–1499
Kjellin H, Johansson H, Höög A, Lehtiö J, Jakobsson P-J, Kjellman M (2014) Differentially Expressed Proteins in Malignant and Benign Adrenocortical Tumors. PLOS ONE 9:e87951
Else T, Lerario AM, Everett J, et al (2017) Adrenocortical carcinoma and succinate dehydrogenase gene mutations. Eur J Endocrinol EJE-17-0358
Ganapathy-Kanniappan S, Geschwind J-FH (2013) Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer 12:152
Warburg O (1956) On the origin of cancer cells. Science 123:309–314
Reske SN, Grillenberger KG, Glatting G, Port M, Hildebrandt M, Gansauge F, Beger HG (1997) Overexpression of glucose transporter 1 and increased FDG uptake in pancreatic carcinoma. J Nucl Med Off Publ Soc Nucl Med 38:1344–1348
Gao Y, Xu D, Yu G, Liang J (2015) Overexpression of metabolic markers HK1 and PKM2 contributes to lymphatic metastasis and adverse prognosis in Chinese gastric cancer. Int J Clin Exp Pathol 8:9264–9271
Wincewicz A, Sulkowska M, Koda M, Sulkowski S (2007) Clinicopathological significance and linkage of the distribution of HIF-1α and GLUT-1 in human primary colorectal cancer. Pathol Oncol Res 13:15
Grover-McKay M, Walsh SA, Seftor EA, Thomas PA, Hendrix MJ (1998) Role for glucose transporter 1 protein in human breast cancer. Pathol Oncol Res POR 4:115–120
Agnihotri S, Zadeh G (2016) Metabolic reprogramming in glioblastoma: the influence of cancer metabolism on epigenetics and unanswered questions. Neuro-Oncol 18:160–172
Takeuchi S, Balachandran A, Habra MA, Phan AT, Bassett RL, Macapinlac HA, Chuang HH (2014) Impact of 18F-FDG PET/CT on the management of adrenocortical carcinoma: analysis of 106 patients. Eur J Nucl Med Mol Imaging 41:2066–2073
Becherer A, Vierhapper H, Pötzi C, Karanikas G, Kurtaran A, Schmaljohann J, Staudenherz A, Dudczak R, Kletter K (2001) FDG-PET in adrenocortical carcinoma. Cancer Biother Radiopharm 16:289–295
Deandreis D, Leboulleux S, Caramella C, Schlumberger M, Baudin E (2011) FDG PET in the management of patients with adrenal masses and adrenocortical carcinoma. Horm Cancer 2:354–362
Robey RB, Hay N (2006) Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene 25:4683–4696
Israelsen WJ, Vander Heiden MG (2015) Pyruvate kinase: Function, regulation and role in cancer. Semin Cell Dev Biol 43:43–51
Jochmanova I, Pacak K (2016) Pheochromocytoma: The First Metabolic Endocrine Cancer. Clin Cancer Res 22:5001–5011
Duan K, Mete O (2017) Familial endocrine tumor syndromes: Clinical and predictive roles of molecular histopathology. AJSP: Reviews and Reports; 22:246–268.
Pacak K, Wimalawansa SJ (2015) Pheochromocytoma and paraganglioma. Endocr Pract 21:406–412
Batisse-Lignier M, Sahut-Barnola I, Tissier F, et al (2017) P53/Rb inhibition induces metastatic adrenocortical carcinomas in a preclinical transgenic model. Oncogene. https://doi.org/10.1038/onc.2017.54
De Martino MC, Feelders RA, de Herder WW, et al (2014) Characterization of the mTOR pathway in human normal adrenal and adrenocortical tumors. Endocr Relat Cancer 21:601–613
Ross JS, Wang K, Rand JV, et al (2014) Next-generation sequencing of adrenocortical carcinoma reveals new routes to targeted therapies. J Clin Pathol 67:968–973
Acknowledgements
The authors would like to thank both Drs. Thomas Giordano and Antonio Marcondes Lerario (University of Michigan Hospital, Michigan Medicine), for assisting the authors to obtain the expression status of HK1, PKM and RPS6 from the TCGA database.
Funding
This work was funded in part by a Canadian Institutes of Health Research (CIHR) New Investigator Foundation Grant FDN-148385 (D.A.W.). D.A.W. holds an Ontario Ministry of Innovation Early Researcher Award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Duan, K., Gucer, H., Kefeli, M. et al. Immunohistochemical Analysis of the Metabolic Phenotype of Adrenal Cortical Carcinoma. Endocr Pathol 31, 231–238 (2020). https://doi.org/10.1007/s12022-020-09624-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-020-09624-3