Abstract
The rhythmic regulation of transcriptional processes is intimately linked to lipid homeostasis, to anticipate daily changes in energy access. The Rev-erbα–HDAC3 complex was previously discovered to execute the rhythmic repression of lipid genes; however, the epigenetic switch that turns on these genes is less clear. Here, we show that genomic recruitment of MRG15, which is encoded by the mortality factor on chromosome 4 (MORF4)-related gene on chromosome 15, displays a significant diurnal rhythm and activates lipid genes in the mouse liver. RNA polymerase II (Pol II) recruitment and histone acetylation correspond to MRG15 binding, and the rhythm is impaired upon MRG15 depletion, establishing MRG15 as a key modulator in global rhythmic transcriptional regulation. MRG15 interacts with the nuclear receptor LRH-1, rather than with known core clock proteins, and is recruited to genomic loci near lipid genes via LRH-1. Blocking of MRG15 by CRISPR targeting or by the FDA-approved drug argatroban, which is an antagonist to MRG15, attenuates liver steatosis. This work highlights MRG15 as a targetable master regulator in the rhythmic regulation of hepatic lipid metabolism.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
ChIP–seq and RNA-seq data have been deposited in the National Omics Data Encylopedia (http://www.biosino.org/node) under the accession code OEP000757.
References
Zhang, R., Lahens, N. F., Ballance, H. I., Hughes, M. E. & Hogenesch, J. B. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc. Natl Acad. Sci. US 111, 16219–16224 (2014).
Takahashi, J. S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 18, 164–179 (2017).
Feng, D. & Lazar, M. A. Clocks, metabolism, and the epigenome. Mol. Cell. 47, 158–167 (2012).
Tao, W. et al. SWItch/sucrose nonfermentable (SWI/SNF) complex subunit BAF60a integrates hepatic circadian clock and energy metabolism. Hepatology 54, 1410–1420 (2011).
Takahata, S. et al. Transactivation mechanisms of mouse clock transcription factors, mClock and mArnt3. Genes Cells 5, 739–747 (2000).
Etchegaray, J. P., Lee, C., Wade, P. A. & Reppert, S. M. Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature 421, 177–182 (2003).
Asher, G. et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 134, 317–328 (2008).
Nakahata, Y. et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134, 329–340 (2008).
DiTacchio, L. et al. Histone lysine demethylase JARID1a activates CLOCK-BMAL1 and influences the circadian clock. Science 333, 1881–1885 (2011).
Doi, M., Hirayama, J. & Sassone-Corsi, P. Circadian regulator CLOCK is a histone acetyltransferase. Cell 125, 497–508 (2006).
Nader, N., Chrousos, G. P. & Kino, T. Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: potential physiological implications. FASEB J. 23, 1572–1583 (2009).
Feng, D. et al. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science 331, 1315–1319 (2011).
Zhang, Y. et al. Discrete functions of nuclear receptor Rev-erbα couple metabolism to the clock. Science 348, 1488–1492 (2015).
Kim, Y. H. et al. Rev-erbα dynamically modulates chromatin looping to control circadian gene transcription. Science 359, 1274–1277 (2018).
Bertram, M. J. & Pereira-Smith, O. M. Conservation of the MORF4 related gene family: identification of a new chromo domain subfamily and novel protein motif. Gene 266, 111–121 (2001).
Chen, M., Pereira-Smith, O. M. & Tominaga, K. Loss of the chromatin regulator MRG15 limits neural stem/progenitor cell proliferation via increased expression of the p21 Cdk inhibitor. Stem Cell Res. 7, 75–88 (2011).
Chen, M., Takano-Maruyama, M., Pereira-Smith, O. M., Gaufo, G. O. & Tominaga, K. MRG15, a component of HAT and HDAC complexes, is essential for proliferation and differentiation of neural precursor cells. J. Neurosci. Res. 87, 1522–1531 (2009).
Garcia, S. N., Kirtane, B. M., Podlutsky, A. J., Pereira-Smith, O. M. & Tominaga, K. Mrg15 null and heterozygous mouse embryonic fibroblasts exhibit DNA-repair defects post exposure to gamma ionizing radiation. FEBS Lett. 581, 5275–5281 (2007).
Huang, C. et al. Mrg15 stimulates Ash1 H3K36 methyltransferase activity and facilitates Ash1 Trithorax group protein function in Drosophila. Nat. Commun. 8, 1649 (2017).
Iwamori, N. et al. MRG15 is required for pre-mRNA splicing and spermatogenesis. Proc. Natl Acad. Sci. USA 113, E5408–E5415 (2016).
Tominaga, K. et al. MRG15 regulates embryonic development and cell proliferation. Mol. Cell. Biol. 25, 2924–2937 (2005).
Pardo, P. S., Leung, J. K., Lucchesi, J. C. & Pereira-Smith, O. M. MRG15, a novel chromodomain protein, is present in two distinct multiprotein complexes involved in transcriptional activation. J. Biol. Chem. 277, 50860–50866 (2002).
Yochum, G. S. & Ayer, D. E. Role for the mortality factors MORF4, MRGX, and MRG15 in transcriptional repression via associations with Pf1, mSin3A, and Transducin-Like Enhancer of Split. Mol. Cell. Biol. 22, 7868–7876 (2002).
Cabianca, D. S. et al. Active chromatin marks drive spatial sequestration of heterochromatin in C. elegans nuclei. Nature 569, 734–739 (2019).
The Coronary Artery Disease (C4D) Genetics Consortium A genome-wide association study in Europeans and South Asians identifies five new loci for coronary artery disease. Nat. Genet. 43, 339–344 (2011).
Reilly, M. P. et al. Identification of ADAMTS7 as a novel locus for coronary atherosclerosis and association of ABO with myocardial infarction in the presence of coronary atherosclerosis: two genome-wide association studies. Lancet 377, 383–392 (2011).
Schunkert, H. et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat. Genet. 43, 333–338 (2011).
Bauer, R. C. et al. Knockout of Adamts7, a novel coronary artery disease locus in humans, reduces atherosclerosis in mice. Circulation 131, 1202–1213 (2015).
Kessler, T. et al. ADAMTS-7 inhibits re-endothelialization of injured arteries and promotes vascular remodeling through cleavage of thrombospondin-1. Circulation 131, 1191–1201 (2015).
Koike, N. et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science 338, 349–354 (2012).
Le Martelot, G. et al. Genome-wide RNA polymerase II profiles and RNA accumulation reveal kinetics of transcription and associated epigenetic changes during diurnal cycles. PLoS Biol. 10, e1001442 (2012).
Zhu, J., Li, C., Gong, C. & Li, X. Regulation of Pol II pausing is involved in daily gene transcription in the mouse liver. J. Biol. Rhythms 33, 350–362 (2018).
Platt, R. J. et al. CRISPR–Cas9 knockin mice for genome editing and cancer modeling. Cell 159, 440–455 (2014).
Pena, A. N., Tominaga, K. & Pereira-Smith, O. M. MRG15 activates the cdc2 promoter via histone acetylation in human cells. Exp. Cell Res. 317, 1534–1540 (2011).
Sun, Z. et al. Hepatic Hdac3 promotes gluconeogenesis by repressing lipid synthesis and sequestration. Nat. Med. 18, 934–942 (2012).
Ueda, H. R. et al. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat. Genet. 37, 187–192 (2005).
Miranda, D. A. et al. LRH-1 regulates hepatic lipid homeostasis and maintains arachidonoyl phospholipid pools critical for phospholipid diversity. JCI Insight 8, e96151 (2018).
Oosterveer, M. H. et al. LRH-1-dependent glucose sensing determines intermediary metabolism in liver. J. Clin. Invest. 122, 2817–2826 (2012).
Stein, S. et al. Impaired SUMOylation of nuclear receptor LRH-1 promotes nonalcoholic fatty liver disease. J. Clin. Invest. 127, 583–592 (2017).
Stein, S. & Schoonjans, K. Molecular basis for the regulation of the nuclear receptor LRH-1. Curr. Opin Cell Biol. 33, 26–34 (2015).
Zou, C. et al. Mortality factor 4 like 1 protein mediates epithelial cell death in a mouse model of pneumonia. Sci. Transl Med. 7, 311ra171 (2015).
Krylova, I. N. et al. Structural analyses reveal phosphatidyl inositols as ligands for the NR5 orphan receptors SF-1 and LRH-1. Cell 120, 343–355 (2005).
Ortlund, E. A. et al. Modulation of human nuclear receptor LRH-1 activity by phospholipids and SHP. Nat. Struct. Mol. Biol. 12, 357–363 (2005).
Wang, W. et al. The crystal structures of human steroidogenic factor-1 and liver receptor homologue-1. Proc. Natl Acad. Sci. USA 102, 7505–7510 (2005).
Li, S. et al. Genetic and chemical screenings identify HDAC3 as a key regulator in hepatic differentiation of human pluripotent stem cells. Stem Cell Reports 11, 22–31 (2018).
Acknowledgements
We thank K. Musunuru and Y. Chen for helpful discussions. We thank H. Feng and X.-F. Chen for assistance with data analysis, and Z.-G. Li for assistance with confocal analysis. This work was supported by grants from the National Key R&D Programme of China (2017YFA0102800 and 2017YFA0103700), the Strategic Priority Research Programme of the Chinese Academy of Sciences (XDA16030402), the National Natural Science Foundation of China (91957205, 31670829 and 31971063) and the China Postdoctoral Science Foundation (2019M661661).
Author information
Authors and Affiliations
Contributions
Y.W., C.T., Y.Z. and Q.D. designed experiments. Y.W., C.T., Y.Z., X.L., F.L., S.L., Y.C., Y.Q., Z.F., L.C, T.Z., X.R., C.F. and Y.L. carried out experiments and analysed data. X.L. performed bioinformatics analysis. Y.W., C.T., Y.Z., F.L., W.Y., H.Y. and Q.D. wrote the manuscript. Q.D. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Primary Handling Editor: Pooja Jha.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Rhythmic genomic recruitment of exogenous MRG15 in mouse liver.
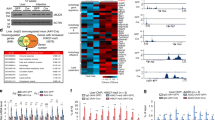
Adeno-associated virus 8 (AAV8) vectors either containing TBG-luciferase (AAV-Luci) or TBG-FLAG-Mrg15 (AAV-FLAG-MRG15) were administered via tail vein injection. Tissues were collected for analysis after virus injection for around 4 weeks. a, Western analysis of endogenous and FLAG-MRG15 levels in mouse liver tissues. Repeated twice with similar results. b, Average MRG15 signals from −5 kb to +5 kb surrounding the TSS and from −2 kb to +2 kb surrounding the gene bodies. c, Distribution of the MRG15 ZT22 binding sites in different genomic locations relative to known genes. d. ChIP–seq tracks of MRG15 signals at ZT10 and ZT22 in Acat2, Acly and Elovl5 gene loci, as indicated. e, Gene enrichment analysis of genes both bound by MRG15 as revealed in ChIP–seq analysis and displayed circadian rhythm at transcriptional levels (603 genes). Associated P values were determined according to the analysis in the GO database (Fisher’s exact P value).
Extended Data Fig. 2 Rhythmic genomic recruitment of endogenous MRG15 in mouse liver in Mrg15Flag-KI animals.
a, Scheme illustration of the generation of the Mrg15Flag-KI animals via knockin of a 3 × FLAG tag at the C-terminal of endogenous Mrg15 before the stop codon. b, Western analysis of the endogenous FLAG-MRG15 with both FLAG and MRG15 antibodies. Repeated twice with similar results. c, Relative MRG15 binding signals as assessed by ChIP–qPCR in animals at ZT10 and ZT22. n = 3 for Wild-type and 6 for Mrg15Flag-KI groups. d, The rhythm of MRG15 recruitment is reversed by daytime feeding. n = 3 for Wild-type, 4 for Mrg15Flag-KI ZT10 and 3 for Mrg15Flag-KI ZT22. Some of the values in the wild-type group are very low and were not detected by qPCR, therefore are not displayed. Data points were all from biologically independent samples (c, d). P values are shown for indicated comparisons by the two-tailed Mann–Whitney U tests. Values are mean ± s.e.m.
Extended Data Fig. 3 Liver-specific depletion of MRG15 via CRISPR technology.
a, Schematic illustration of the CRISPR–Cas9 KI mice and AAV constructs used to deplete genes in this study. b, In vivo bioluminescence imaging of AAV vectors (left) and endogenous liver MRG15 levels in animals administered AAV-Cre-2a-Luci (Control) or AAV-Cre-2a-Luci-Mrg15 sgRNA1 (Mrg15-sgRNA). Repeated twice with similar results.
Extended Data Fig. 4 MRG15 depletion in liver leads to reduced expression of lipid and cholesterol synthesis genes in NCD-fed animals.
Tissues were collected from animals under NCD after virus injection for 14 weeks for RNA-seq analysis. a, b, Representative genes downregulated in CRISPR-Mrg15 livers involved in triglyceride synthesis (a) and cholesterol synthesis (b). Values are indicated as log 2 [Fold change (CRISPR-Mrg15/Control)] in a and b. Genes that are direct targets of MRG15 as revealed in ChIP–seq analysis are labelled with an asterisk at upper right.
Extended Data Fig. 5 MRG15 binding sites show minimal overlap with binding loci of HDAC3, Rev-erbα or NCoR, at commonly bound genes.
Heatmap displaying localisation of ChIP signals relative to the TSS site (0 bp) near common target genes between MRG15 vs. HDAC3 a, MRG15 vs. Rev-erbα b, and MRG15 vs. NCoR c. The numbers of common target genes and overlapped peaks are indicated below, with common genes (peaks) showing in blue, and total bound genes (peaks) of HDAC3, Rev-erbα and NCoR are shown in black. Detailed information can also be found in Supplementary Table 1.
Extended Data Fig. 6 LRH-1 and MRG15 co-regulate lipid synthesis genes.
a, Venn diagram indicating overlapping genes in Lrh1−/− and CRISPR-Mrg15 livers (left); and gene enrichment analysis of overlapped genes downregulated in Lrh1−/− and CRISPR-Mrg15 livers (120 genes). b, Venn diagram indicating overlapping genes in LRH-1 K289R and CRISPR-Mrg15 livers (left); and gene enrichment analysis of overlapped genes upregulated in LRH-1 K289R livers and downregulated in CRISPR-Mrg15 livers (161 genes). Associated P values were determined according to the analysis in the GO database (Fisher’s exact P value) for a and b. c, Representative commonly regulating lipid synthesis genes between Lrh1−/− and CRISPR-Mrg15 livers, and LRH-1 K289R and CRISPR-Mrg15 livers.
Extended Data Fig. 7 mRNA expression levels of Mrg15 and Lrh-1 at ZT10 and ZT22.
Relative mRNA expression of Mrg15 and Lrh-1 in liver at ZT10 and ZT22. n = 5 biologically independent samples. P values are shown for indicated comparisons by the two-tailed Mann–Whitney U tests. Values are mean ± s.e.m.
Extended Data Fig. 8 Liver depletion of MRG15 leads to reduced expression of lipid and cholesterol synthesis genes, and inflammatory genes in HFD animals.
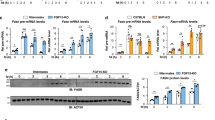
Tissues were collected from animals under HFD after virus injection for 14 weeks for RNA-seq analysis. a, b, c, Representative genes downregulated in CRISPR-Mrg15 livers involved in triglyceride synthesis (a), cholesterol synthesis (b) and inflammatory response (c). Values are indicated as log 2 [Fold change (CRISPR-Mrg15/Control)] in a, b and c. Genes that are direct targets of MRG15 as revealed in ChIP–seq analysis in a and b are labelled with an asterisk at upper right. d, Gene enrichment analysis of downregulated genes in CRISPR-Mrg15 livers identified by RNA-seq (622 genes using a cut-off of P < 0.05). Associated P values were determined according to the analysis in the GO database (Fisher’s exact P value). e, Relative mRNA expression of representative lipid genes in liver in control and Mrg15-sgRNA treated animals under HFD. n = 9 biologically independent samples. P values are shown for indicated comparisons by the two-tailed Mann–Whitney U tests. Values are mean ± s.e.m.
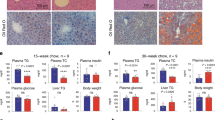
Extended Data Fig. 9 CRISPR-Mrg15-sgRNA2 mice have improved metabolism.
a, Mrg15 targeting sequences of two sgRNAs. b, Western analysis of endogenous MRG15 levels after CRISPR treatment. Repeated twice with similar results. c, Body weight. d, Nuclear magnetic resonance analysis of fat mass. e, Nuclear magnetic resonance analysis of lean mass. Start, before virus injection. End, 19 weeks after virus injection. n = 10 (a-e). f, Blood triglyceride levels and total cholesterol levels (1 month: n = 11 and 13; 2 months: n = 13 and 15). g, GTT and ITT of animals after virus injection for 9–10 weeks. n = 11 for Control and 13 for Mrg15-sgRNA 2. h, Western analysis of endogenous MRG15 and LRH-1 levels after CRISPR treatment. Repeated twice with similar results. Data points were all from biologically independent samples (c-g). P values are shown for indicated comparisons by the two-tailed Mann–Whitney U tests (f) or were determined by multiple t-tests with fewer assumptions (do not assume consistent s.d.) for GTT and ITT analyses (g). Values are mean ± s.e.m.
Extended Data Fig. 10 MRG15 regulates expression of several core clock genes.
Relative mRNA levels of core clock genes in animal livers over a 24-h cycle. n = 4 biologically independent samples. Values are mean ± s.e.m.
Supplementary information
Supplementary Information
Supplementary Figures 1–4 and Supplementary Table 1
Supplementary Table 1
Statistical Source Data Supplementary Fig. 3
Source data
Source Data Fig. 1
Statistical Source Data
Source Data Fig. 2
Statistical Source Data
Source Data Fig. 3
Statistical Source Data
Source Data Fig. 4
Statistical Source Data
Source Data Fig. 5
Statistical Source Data
Source Data Fig. 5
Unprocessed Western Blots
Source Data Fig. 6
Statistical Source Data
Source Data Fig. 7
Statistical Source Data
Source Data Fig. 7
Unprocessed Western Blots
Source Data Extended Data Fig. 1
Unprocessed Western Blots
Source Data Extended Data Fig. 2
Statistical Source Data
Source Data Extended Data Fig. 2
Unprocessed Western Blots
Source Data Extended Data Fig. 3
Unprocessed Western Blots
Source Data Extended Data Fig. 7
Statistical Source Data
Source Data Extended Data Fig. 8
Statistical Source Data
Source Data Extended Data Fig. 9
Statistical Source Data,
Source Data Extended Data Fig. 9
Unprocessed Western Blots
Source Data Extended Data Fig. 10
Statistical Source Data
Rights and permissions
About this article
Cite this article
Wei, Y., Tian, C., Zhao, Y. et al. MRG15 orchestrates rhythmic epigenomic remodelling and controls hepatic lipid metabolism. Nat Metab 2, 447–460 (2020). https://doi.org/10.1038/s42255-020-0203-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-020-0203-z
This article is cited by
-
Structural and functional insights into the epigenetic regulator MRG15
Acta Pharmacologica Sinica (2024)
-
Acute liver steatosis translationally controls the epigenetic regulator MIER1 to promote liver regeneration in a study with male mice
Nature Communications (2023)
-
ADAMTS7: a Novel Therapeutic Target in Atherosclerosis
Current Atherosclerosis Reports (2023)