Abstract
Purpose
To develop a new protocol for whole-ovary decellularization for the production of a 3D bioscaffold suitable for in vitro/ex vivo studies and for the reconstruction of a bioengineered ovary.
Methods
Porcine ovaries were subjected to the decellularization process (DECELL; n = 20) that involved a freeze-thaw cycle, followed by sequential incubations in 0.5% SDS for 3 h, 1% Triton X-100 for 9 h, and 2% deoxycholate for 12 h. Untreated ovaries were used as a control (CTR; n = 6). Both groups were analyzed to evaluate cell and DNA removal as well as ECM preservation. DECELL bioscaffolds were assessed for cytotoxicity and cell homing ability.
Results
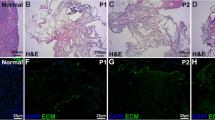
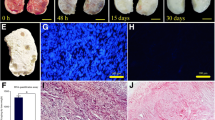
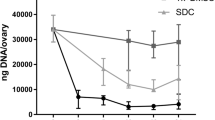
DECELL ovaries maintained shape and homogeneity without any deformation, while their color turned from red to white. Histological staining and DNA quantification confirmed a decrease of 98.11% in DNA content, compared with the native tissue (CTR). Histochemical assessments demonstrated the preservation of intact ECM microarchitecture after the decellularization process. This was also confirmed by quantitative analysis of collagen, elastin, and GAG contents. DECELL bioscaffold showed no cytotoxic effects in co-culture and, when re-seeded with homologous fibroblasts, encouraged a rapid cell adhesion and migration, with repopulating cells increasing in number and aggregating in cluster-like structures, consistent with its ability to sustain cell adherence, proliferation, and differentiation.
Conclusion
The protocol described allows for the generation of a 3D bioscaffold that may constitute a suitable model for ex vivo culture of ovarian cells and follicles, as well as a promising tool for the reconstruction of a bioengineered ovary.




Similar content being viewed by others
Change history
28 July 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10815-023-02894-w
References
Hewlett M, Mahalingaiah S. Update on primary ovarian insufficiency. Curr Opin Endocrinol Diabetes Obes. 2015;22:483–9.
Qin Y, Jiao X, Simpson JL, Chen Z-J. Genetics of primary ovarian insufficiency: new developments and opportunities. Hum Reprod Update. 2015;21:787–808.
Gandolfi F, Ghiringhelli M, Brevini TAL, Gandolfi F, Ghiringhelli M, Brevini TAL. Bioengineering the ovary to preserve and reestablish female fertility. Anim Reprod. 2019;16:45–51.
Trinh X-B, Peeters F, Tjalma WAA. The thoughts of breast cancer survivors regarding the need for starting hormone replacement therapy. Eur J Obstet Gynecol Reprod Biol. 2006;124:250–3.
Yalcinkaya TM, Sittadjody S, Opara EC. Scientific principles of regenerative medicine and their application in the female reproductive system. Maturitas. 2014;77:12–9.
Sadri-Ardekani H, Atala A. Regenerative medicine for the treatment of reproductive system disorders: current and potential options. Adv Drug Deliv Rev. 2015;82–83:145–52.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34.
Vajta G, Kuwayama M. Improving cryopreservation systems. Theriogenology. 2006;65:236–44.
Wong KM, Mastenbroek S, Repping S. Cryopreservation of human embryos and its contribution to in vitro fertilization success rates. Fertil Steril. 2014;102:19–26.
Sparks A. Human embryo cryopreservation—methods, timing, and other considerations for optimizing an embryo cryopreservation program. Semin Reprod Med. 2015;33:128–44.
Chen C. Pregnancy after human oocyte cryopreservation. Lancet. 1986;327:884–6.
Westphal LM, Massie JAM, Lentscher JA. Embryo and oocyte banking. Textb Oncofertility Res Pract. 2019. p. 71–9.
Porcu E. Oocyte freezing. Semin Reprod Med. 2001;19:221–30.
Davis VJ. Female gamete preservation. Cancer. 2006;107:1690–4.
Gook DA, Edgar DH. Cryopreservation of female reproductive potential. Best Pract Res Clin Obstet Gynaecol. 2019;55:23–36.
Donnez J, Dolmans M-M, Pellicer A, Diaz-Garcia C, Sanchez Serrano M, Schmidt KT, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99:1503–13.
Donnez J, Dolmans M-M. Fertility preservation in women. Nat Rev Endocrinol. 2013;9:735–49.
Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, et al. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30:608–15.
Maffei S, Hanenberg M, Pennarossa G, Silva JRV, Brevini TAL, Arav A, et al. Direct comparative analysis of conventional and directional freezing for the cryopreservation of whole ovaries. Fertil Steril. 2013;100:1122–31.
Maffei S, Pennarossa G, Brevini TAL, Arav A, Gandolfi F. Beneficial effect of directional freezing on in vitro viability of cryopreserved sheep whole ovaries and ovarian cortical slices. Hum Reprod. 2014;29:114–24.
Moffa F, Biacchiardi CP, Fagioli F, Biasin E, Revelli A, Massobrio M, et al. Ovarian tissue cryostorage and grafting: an option to preserve fertility in pediatric patients with malignancies. Pediatr Hematol Oncol. 2007;24:29–44.
Hutt KJ, Albertini DF. An oocentric view of folliculogenesis and embryogenesis. Reprod BioMed Online. 2007;14:758–64.
Meirow D, Hardan I, Dor J, Fridman E, Elizur S, Ra’anani H, et al. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Hum Reprod. 2008;23:1007–13.
Dolmans MM, Marinescu C, Saussoy P, Van Langendonckt A, Amorim C, Donnez J. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood. 2010;116:2908–14.
Greve T, Clasen-Linde E, Andersen MT, Andersen MK, Sørensen SD, Rosendahl M, et al. Cryopreserved ovarian cortex from patients with leukemia in complete remission contains no apparent viable malignant cells. Blood. 2012;120:4311–6.
Gilpin A, Yang Y. Decellularization strategies for regenerative medicine: from processing techniques to applications. Biomed Res Int. 2017;2017:9831534.
Porzionato A, Stocco E, Barbon S, Grandi F, Macchi V, De Caro R. Tissue-engineered grafts from human decellularized extracellular matrices: a systematic review and future perspectives. Int J Mol Sci. 2018;19.
Rajabi-Zeleti S, Jalili-Firoozinezhad S, Azarnia M, Khayyatan F, Vahdat S, Nikeghbalian S, et al. The behavior of cardiac progenitor cells on macroporous pericardium-derived scaffolds. Biomaterials. 2014;35:970–82.
Lecht S, Stabler CT, Rylander AL, Chiaverelli R, Schulman ES, Marcinkiewicz C, et al. Enhanced reseeding of decellularized rodent lungs with mouse embryonic stem cells. Biomaterials. 2014;35:3252–62.
Lee H, Han W, Kim H, Ha D-H, Jang J, Kim BS, et al. Development of liver decellularized extracellular matrix bioink for three-dimensional cell printing-based liver tissue engineering. Biomacromolecules. 2017;18:1229–37.
Yu YL, Shao YK, Ding YQ, Lin KZ, Chen B, Zhang HZ, et al. Decellularized kidney scaffold-mediated renal regeneration. Biomaterials. 2014;35:6822–8.
Aulino P, Costa A, Chiaravalloti E, Perniconi B, Adamo S, Coletti D, et al. Muscle extracellular matrix scaffold is a multipotent environment. Int J Med Sci. 2015;12:336–40.
Baiguera S, Del Gaudio C, Kuevda E, Gonfiotti A, Bianco A, Macchiarini P. Dynamic decellularization and cross-linking of rat tracheal matrix. Biomaterials. 2014;35:6344–50.
Sjöqvist S, Jungebluth P, Lim ML, Haag JC, Gustafsson Y, Lemon G, et al. Experimental orthotopic transplantation of a tissue-engineered oesophagus in rats. Nat Commun. 2014;5:3562.
Singh A, Bivalacqua TJ, Sopko N. Urinary tissue engineering: challenges and opportunities. Sex Med Rev. 2018;6:35–44.
Kajbafzadeh A-M, Khorramirouz R, Kameli SM, Hashemi J, Bagheri A. Decellularization of human internal mammary artery: biomechanical properties and histopathological evaluation. Biores Open Access. 2017;6:74–84.
Zhang J-K, Du R-X, Zhang L, Li Y-N, Zhang M-L, Zhao S, et al. A new material for tissue engineered vagina reconstruction: Acellular porcine vagina matrix. J Biomed Mater Res Part A. 2017;105:1949–59.
Laronda MM, Rutz AL, Xiao S, Whelan KA, Duncan FE, Roth EW, et al. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat Commun. 2017;8:15261.
Laronda MM, Jakus AE, Whelan KA, Wertheim JA, Shah RN, Woodruff TK. Initiation of puberty in mice following decellularized ovary transplant. Biomaterials. 2015;50:20–9.
Pors SE, Ramløse M, Nikiforov D, Lundsgaard K, Cheng J, Andersen CY, et al. Initial steps in reconstruction of the human ovary: survival of pre-antral stage follicles in a decellularized human ovarian scaffold. Hum Reprod. 2019;34:1523–35.
Liu W-Y, Lin S-G, Zhuo R-Y, Xie Y-Y, Pan W, Lin X-F, et al. Xenogeneic decellularized scaffold: a novel platform for ovary regeneration. Tissue Eng Part C Methods. 2017;23:61–71.
Hassanpour A, Talaei-Khozani T, Kargar-Abarghouei E, Razban V, Vojdani Z. Decellularized human ovarian scaffold based on a sodium lauryl ester sulfate (SLES)-treated protocol, as a natural three-dimensional scaffold for construction of bioengineered ovaries. Stem Cell Res Ther. 2018;9:252.
Eivazkhani F, Abtahi NS, Tavana S, Mirzaeian L, Abedi F, Ebrahimi B, et al. Evaluating two ovarian decellularization methods in three species. Mater Sci Eng C. 2019;102:670–82.
Alshaikh AB, Padma AM, Dehlin M, Akouri R, Song MJ, Brännström M, et al. Decellularization of the mouse ovary: comparison of different scaffold generation protocols for future ovarian bioengineering. J Ovarian Res. 2019;12:58.
Bondioli K, Ramsoondar J, Williams B, Costa C, Fodor W. Cloned pigs generated from cultured skin fibroblasts derived from a H-transferase transgenic boar. Mol Reprod Dev. 2001;60:189–95.
Oktem O, Oktay K. The role of extracellular matrix and activin-A in in vitro growth and survival of murine preantral follicles. Reprod Sci. 2007;14:358–66.
Oktay K, Karlikaya G, Akman O, Ojakian GK, Oktay M. Interaction of extracellular matrix and activin-A in the initiation of follicle growth in the mouse ovary. Biol Reprod. 2000;63:457–61.
Laronda MM. Engineering a bioprosthetic ovary for fertility and hormone restoration. Theriogenology. 2020;S0093-691X(20):30027–3.
O’Neill JD, Anfang R, Anandappa A, Costa J, Javidfar J, Wobma HM, et al. Decellularization of human and porcine lung tissues for pulmonary tissue engineering. Ann Thorac Surg. 2013;96:1046–56.
Zhou J, Fritze O, Schleicher M, Wendel H-P, Schenke-Layland K, Harasztosi C, et al. Impact of heart valve decellularization on 3-D ultrastructure, immunogenicity and thrombogenicity. Biomaterials. 2010;31:2549–54.
Uygun BE, Soto-Gutierrez A, Yagi H, Izamis M-L, Guzzardi MA, Shulman C, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814–20.
Henning NF, LeDuc RD, Even KA, Laronda MM. Proteomic analyses of decellularized porcine ovaries identified new matrisome proteins and spatial differences across and within ovarian compartments. Sci Rep. 2019;9:20001.
Morris AH, Stamer DK, Kyriakides TR. The host response to naturally-derived extracellular matrix biomaterials. Semin Immunol. 2017;29:72–91.
Laronda MM, Burdette JE, Kim J, Woodruff TK. Recreating the female reproductive tract in vitro using iPSC technology in a linked microfluidics environment. Stem Cell Res Ther. 2013;4:S13.
Brevini TAL, Pennarossa G, Gandolfi F. A 3D approach to reproduction. Theriogenology. 2020;S0093-691X(20):30026–1.
Acknowledgments
The Laboratory of Biomedical Embryology is a member of the COST Action CA16119 In vitro 3-D total cell guidance and fitness (CellFit). SEM analysis was carried out at NOLIMITS, an advanced imaging facility established by the Università degli Studi di Milano.
Funding
This work was funded by Carraresi Foundation and PSR2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Fig. 4 in the original version of this article has been replaced.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pennarossa, G., Ghiringhelli, M., Gandolfi, F. et al. Whole-ovary decellularization generates an effective 3D bioscaffold for ovarian bioengineering. J Assist Reprod Genet 37, 1329–1339 (2020). https://doi.org/10.1007/s10815-020-01784-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-01784-9