Abstract
Purpose
Loss-in-weight feeders play a vital role in assuring blend and content uniformity as well as lot-to-lot powder traceability in continuous manufacturing. Irregular flow from the feeders propagates through the system, potentially resulting in out-of-specification product. Powder properties such as density, cohesion, and adhesion can cause large variability in the flow rate of ingredients from powder feeders. Feeding of active pharmaceutical ingredients (API) can be difficult because of inherently poor flow, low density, high cohesion, and adhesion.
Method
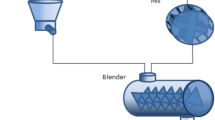
API was chosen due to known adhesion behavior inside feeders during continuous operations. The selected APIs were blended with nanosized silica, in a V-blender, to provide separation between the API particles and reducing the effect of interparticle forces. The material was characterized by standard pharmaceutical techniques to identify bulk changes between the as-received and the blended API. The coated API was then fed using a Coperion K-Tron KT-20 pharmaceutical loss-in-weight feeder. The material was dispensed onto a catch scale, recording mass versus time, and analyzed for relative standard deviation and deviation from the set point. Additionally, the mass of the remaining API in the feeder after the run ended was compared between the API and silica-blended API.
Results
Blending APIs with nanosized silica successfully improved API feeding performance despite their intrinsic highly adhesive and cohesive behavior. Both the pure and silica-blended APIs were fed using a twin screw powder feeder. When feeding unsilicated APIs, large variability in flow rate and screw speed were observed, and large amounts of material coated the inside of the hopper and screws. When the adhesive APIs were blended with nanosized silica and fed through a loss-in-weight feeder, there was significantly less adhesion to the feeder, and the material was dispensed with significant reduction in flow rate and screw speed variability.
Conclusions
This work provides a means to increase the applicability of continuous manufacturing by enabling the practitioner to manage adverse impact of cohesive material properties on feeder performance. Blending highly adhesive API with silica reduces the adhesion of the API to the feeder hopper and screws, while also improving the deviation in mass flow rate exiting the feeder.








Similar content being viewed by others
References
Ierapetritou MG, Muzzio FJ, Reklaitis G. Perspectives on the continuous manufacturing of powder-based pharmaceutical processes. AICHE J. 2016;62(6):1846–62.
Byrn S, Futran M, Thomas H, Jayjock E, Maron N, Meyer RF, et al. Achieving continuous manufacturing for final fosage formation: challenges and how to meet them. J Pharm Sci. 2015;104(3):792–802.
Kleinebudde P, Khinast J, Rantanen J. Continuous manufacturing of pharmaceuticals, vol. 7703. Wiley; 2017.
Lee SL, O’Connor TF, Yang X, Cruz CN, Chatterjee S, Madurawe RD, et al. Modernizing pharmaceutical manufacturing: from batch to continuous production. J Pharm Innov. 2015;10(3):191–9.
Ammarcha C, Gatumel C, Dirion JL, Cabassud M, Berthiaux H. Continuous powder mixing of segregating mixtures under steady and unsteady state regimes: homogeneity assessment by real-time on-line image analysis. Powder Technol. 2017;315(Supplement C):39–52.
Marikh K, Berthiaux H, Gatumel C, Mizonov V, Barantseva E. Influence of stirrer type on mixture homogeneity in continuous powder mixing: a model case and a pharmaceutical case. Chem Eng Res Des. 2008;86(9A):1027–37.
Oka S, Sahay A, Meng W, Muzzio FJ. Diminished segregation in continuous powder mixing. Powder Technol. 2017;309(Supplement C):79–88.
Martínez L, Peinado A, Liesum L, Betz G. Use of near-infrared spectroscopy to quantify drug content on a continuous blending process: influence of mass flow and rotation speed variations. Eur J Pharm Biopharm. 2013;84(3):606–15.
Roth WJ, Almaya A, Kramer TT, Hofer JD. A demonstration of mixing robustness in a direct compression continuous manufacturing process. J Pharm Sci. 2017;106(5):1339–46.
Van Snick B, Holman J, Vanhoorne V, Kumar A, De Beer T, Remon JP, et al. Development of a continuous direct compression platform for low-dose drug products. Int J Pharm. 2017;529(1–2):329–46.
Berthiaux H, Marikh K, Gatumel C. Continuous mixing of powder mixtures with pharmaceutical process constraints. Chem Eng Process. 2008;47(12):2315–22.
Ervasti T, Simonaho SP, Ketolainen J, Forsberg P, Fransson M, Wikström H, et al. Continuous manufacturing of extended release tablets via powder mixing and direct compression. Int J Pharm. 2015;495(1):290–301.
Van Snick B, Holman J, Cunningham C, Kumar A, Vercruysse J, De Beer T, et al. Continuous direct compression as manufacturing platform for sustained release tablets. Int J Pharm. 2017;519(1):390–407.
Vanarase AU, Alcalà M, Rozo JIJ, Muzzio FJ, Romañach RJ. Real-time monitoring of drug concentration in a continuous powder mixing process using NIR spectroscopy. Chem Eng Sci. 2010;65(21):5728–33.
Vanarase AU, Osorio JG, Muzzio FJ. Effects of powder flow properties and shear environment on the performance of continuous mixing of pharmaceutical powders. Powder Technol. 2013;246:63–72.
Weinekötter R, Gericke H. Mixing of solids. Netherlands: Springer; 2013.
Blackshields CA, Crean AM. Continuous powder feeding for pharmaceutical solid dosage form manufacture: a short review. Pharm Dev Technol. 2018;23(6):554–60.
Ierapetritou MG, Escotet-Espinoza MS, Singh R. Process simulation and control for continuous pharmaceutical manufacturing of solid drug products. In: Continuous Manufacturing of Pharmaceuticals; 2017. p. 33–105.
Oka S, Escotet-Espinoza MSS, Singh R, Scicolone JV, Hausner DB, Ierapetritou MG, et al. Design of an integrated continuous manufacturing system. In: Continuous Manufacturing of Pharmaceuticals; 2017. p. 405–46.
Engisch WE, Muzzio FJ. Loss-in-weight feeding trials case study: pharmaceutical formulation. J Pharm Innov. 2014;10(1):56–75.
Moghtadernejad S, Escotet-Espinoza MS, Oka S, Singh R, Liu Z, Román-Ospino AD, et al. A training on: continuous manufacturing (direct compaction) of solid dose pharmaceutical products. J Pharm Innov. 2018;13(2):155–87.
Escotet-Espinoza MS, Singh R, Sen M, O’connor T, Lee S, Chatterjee S, et al. Flowsheet models modernize pharmaceutical manufacturing design and risk assessment. Pharm Technol. 2015;39(4):34–42.
Wang Z, Escotet-Espinoza MS, Ierapetritou MG. Process analysis and optimization of continuous pharmaceutical manufacturing using flowsheet models. Comput Chem Eng. 2017;107:77–91.
Escotet-Espinoza MS. Phenomenological and residence time distribution models for unit operations in a continuous pharmaceutical manufacturing process. In: Chemical and Biochemical Engineering: Rutgers, The State University of New Jersey; 2018.
García-Muñoz S, Butterbaugh A, Leavesley I, Manley LF, Slade D, Bermingham S. A flowsheet model for the development of a continuous process for pharmaceutical tablets: an industrial perspective. AICHE J. 2018;64:511–25.
Almaya A, De Belder L, Meyer R, Nagapudi K, Lin HH, Leavesley I, et al. Control strategies for drug product continuous direct compression-state of control, product collection strategies, and startup/shutdown operations for the production of clinical trial materials and commercial products. J Pharm Sci. 2017;106(4):930–43.
Boukouvala F, Niotis V, Ramachandran R, Muzzio FJ, Ierapetritou MG. An integrated approach for dynamic flowsheet modeling and sensitivity analysis of a continuous tablet manufacturing process. Comput Chem Eng. 2012;42:30–47.
Simonaho SP, Ketolainen J, Ervasti T, Toiviainen M, Korhonen O. Continuous manufacturing of tablets with PROMIS-line — introduction and case studies from continuous feeding, blending and tableting. Eur J Pharm Sci. 2016;90(Supplement C):38–46.
Wang Y, Li T, Muzzio FJ, Glasser BJ. Predicting feeder performance based on material flow properties. Powder Technol. 2017;308:135–48.
Van Snick B, et al. Impact of material properties and process variables on the residence time distribution in twin screw feeding equipment. Int J Pharm. 2019;556:200–16.
Escotet-Espinoza MS, Sara M, Scicolone J, Wang Y, Pereira G, Schäfer E, et al. Using a material property library to find surrogate materials for pharmaceutical process development. Powder Technol. 2018;339:659–79.
Willecke N, Szepes A, Wunderlich M, Remon JP, Vervaet C, De Beer T. A novel approach to support formulation design on twin screw wet granulation technology: understanding the impact of overarching excipient properties on drug product quality attributes. Int J Pharm. 2018;545(1):128–43.
Escotet-Espinoza MS, Vadodaria S, Singh R, Muzzio FJ, Ierapetritou MG. Modeling the effects of material properties on tablet compaction: a building block for controlling both batch and continuous pharmaceutical manufacturing processes. Int J Pharm. 2018;543(1):274–87.
Zworku ZA, Kumar D, Gomes JV, He Y, Glennon B, Ramisetty KA, et al. Modelling and understanding powder flow properties and compactability of selected active pharmaceutical ingredients, excipients and physical mixtures from critical material properties. Int J Pharm. 2017;531(1):191–204.
Engisch WE, Muzzio FJ. Method for characterization of loss-in-weight feeder equipment. Powder Technol. 2012;228:395–403.
Chen L, Ding X, He Z, Fan S, Kunnath KT, Zheng K, et al. Surface engineered excipients: II. Simultaneous milling and dry coating for preparation of fine-grade microcrystalline cellulose with enhanced properties. Int J Pharm. 2018;546(1):125–36.
Chen L, Ding X, He Z, Huang Z, Kunnath KT, Zheng K, et al. Surface engineered excipients: I. Improved functional properties of fine grade microcrystalline cellulose. Int J Pharm. 2018;536(1):127–37.
Ghoroi C, Gurumurthy L, McDaniel DJ, Jallo LJ, Davé RN. Multi-faceted characterization of pharmaceutical powders to discern the influence of surface modification. Powder Technol. 2013;236:63–74.
Han X, Ghoroi C, Davé R. Dry coating of micronized API powders for improved dissolution of directly compacted tablets with high drug loading. Int J Pharm. 2013;442(1):74–85.
Han X, Jallo L, To D, Ghoroi C, Davé R. Passivation of high-surface-energy sites of milled ibuprofen crystals via dry coating for reduced cohesion and improved flowability. J Pharm Sci. 2013;102(7):2282–96.
Huang Z, Xiong W, Kunnath K, Bhaumik S, Davé RN. Improving blend content uniformity via dry particle coating of micronized drug powders. Eur J Pharm Sci. 2017;104:344–55.
Kojima T, Elliott JA. Effect of silica nanoparticles on the bulk flow properties of fine cohesive powders. Chem Eng Sci. 2013;101:315–28.
Mullarney MP, Beach LE, Davé RN, Langdon BA, Polizzi M, Blackwood DO. Applying dry powder coatings to pharmaceutical powders using a comil for improving powder flow and bulk density. Powder Technol. 2011;212(3):397–402.
Zhou Q, Denman JA, Gengenbach T, Das S, Qu L, Zhang H, et al. Characterization of the surface properties of a model pharmaceutical fine powder modified with a pharmaceutical lubricant to improve flow via a mechanical dry coating approach. J Pharm Sci. 2011;100(8):3421–30.
Jallo LJ, Chen Y, Bowen J, Etzler F, Dave R. Prediction of inter-particle adhesion force from surface energy and surface roughness. J Adhes Sci Technol. 2011;25(4–5):367–84.
Jallo LJ, Schoenitz M, Dreizin EL, Dave RN, Johnson CE. The effect of surface modification of aluminum powder on its flowability, combustion and reactivity. Powder Technol. 2010;204(1):63–70.
Tran DT, Majerová D, Veselý M, Kulaviak L, Ruzicka MC, Zámostný P. On the mechanism of colloidal silica action to improve flow properties of pharmaceutical excipients. Int J Pharm. 2019;556:383–94.
Rowe R, Sheskey P, Owen S. In: R.P.S.o.G. Britain, editor. Handbook of Pharmaceutical Excipients. 5th ed. London: Pharmaceutical Press and the American Pharmacists Association; 2006.
Huang Z, Scicolone JV, Han X, Davé RN. Improved blend and tablet properties of fine pharmaceutical powders via dry particle coating. Int J Pharm. 2015;478(2):447–55.
Lee Y-C, McNevin M, Ikeda C, Chouzouri G, Moser J, Harris D, et al. Combination of colloidal silicon dioxide with spray-dried solid dispersion to facilitate discharge from an agitated dryer. AAPS PharmSciTech. 2019;20(5):182.
NIST/SEMATECH, Engineering Statistics Handbook, in Process or Product Monitoring and Controls - Time Series Analysis - Single Moving Average. 2012, US Department of Commerce: Online.
Mullarney MP, Beach LE, Davé RN, Langdon BA, Polizzi M, Blackwood DO. Applying dry powder coatings to pharmaceutical powders using a comil for improving powder flow and bulk density. Powder Technol. 2011;212(3):397–402.
Yang J, Sliva A, Banerjee A, Dave RN, Pfeffer R. Dry particle coating for improving the flowability of cohesive powders. Powder Technol. 2005;158(1):21–33.
Huang Z, Scicolone JV, Gurumuthy L, Davé RN. Flow and bulk density enhancements of pharmaceutical powders using a conical screen mill: a continuous dry coating device. Chem Eng Sci. 2015;125:209–24.
Osorio JG, Muzzio FJ. Effects of powder flow properties on capsule filling weight uniformity. Drug Dev Ind Pharm. 2013;39(9):1464–75.
Li T, Scicolone JV, Sanchez E, Muzzio FJ. Identifying a loss-in-weight feeder design space based on performance and material properties. J Pharm Innov. 2019:1–14.
Acknowledgments
The authors would like to thank Tianyi Li, Kien Chau, Sejal Shah, and Glinka Pereira for their assistance in this work.
Funding
The research was financially supported by the Janssen Ortho LLC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
M. Sebastian Escotet-Espinoza and James V. Scicolone share equal first authorship.
Rights and permissions
About this article
Cite this article
Escotet-Espinoza, M.S., Scicolone, J.V., Moghtadernejad, S. et al. Improving Feedability of Highly Adhesive Active Pharmaceutical Ingredients by Silication. J Pharm Innov 16, 279–292 (2021). https://doi.org/10.1007/s12247-020-09448-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-020-09448-y