Abstract
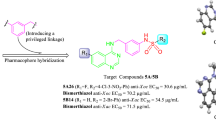
The objective of this study was to evaluate the synthesis, structural analysis, and biological effects of novel ((2, 4-dioxothiazolidin-5-ylidene)methyl)phenyl derivatives. The efficacy of 15-PGDH inhibition increased for the substituents in the derivatives in the order: cyclohexylpropyl > cyclohexylethyl > cyclohexylmethyl > cyclohexyl. Compound 12 inhibited 15-PGDH activity by binding to the amino acids Ile 214, Ile 210, Ile 194, Gln 148, and Leu 191 of 15-PGDH. Compounds 4, 22, and 23 produced the highest increment in PGE2 concentration, which was 122.19, 100.14, and 206.80%, respectively, compared to that of the control. Also, compounds 38 and 39, in which the central phenyl ring and the 2, 4-thiazolidinedione moiety were linked by a single bond, produced a relatively high increment in PGE2 concentration, which was 106.81 and 118.66%, respectively, compared to that of the control. The wound closure rates of compounds 22 and 39 were the highest, being 247.56 and 202.42%, respectively, compared to that of the positive control. Therefore, compounds 22 and 39 could not only efficiently regulate PGE2 concentration, but also induce cellular regeneration, and are expected to effectively treat a variety of diseases resulting from PGE2 deficiency.
Similar content being viewed by others
References
Martin, P. (1997) Wound healing, aiming for perfect skin regeneration. Science. 276: 75–81.
Diegelmann, R. F. and M. C. Evans (2004) Wound healing: An overview of acute, fibrotic and delayed healing. Front Biosci. 9: 283–289.
Ricciotti, E. and G. A. FitzGerald (2011) Prostaglandins and inflammation. Arterioscler Thromb. Vasc. Biol. 31: 986–1000.
Loynes, C. A., J. A. Lee, A. L. Robertson, M. J. Steel, F. Ellett, Y. Feng, B. D. Levy, M. K. Whyte, and S. A. Renshaw (2018) PGE2 production at sites of tissue injury promotes an anti-inflammatory neutrophil phenotype and determines the outcome of inflammation resolution in vivo. Sci. Adv. 4: eaar8320.
Kalinski, P. (2012) Regulation of immune responses by prostaglandin E2. J. Immunol. 188: 21–28.
Antczak, M. I., Y. Zhang, C. Wang, J. Doran, J. Naidoo, S. Voruganti, N. S. Williams, S. D. Markowitz, and J. M. Ready (2017) Inhibitors of 15-prostaglandin dehydrogenase to potentiate tissue repair. J. Med. Chem. 60: 3979–4001.
Samuelsson. B., R. Morgenstern, and P. J. Jakobsson (2007) Membrane prostaglandin E synthase-1: a novel therapeutic target. Pharmacol. Rev. 59: 207–224.
Kawahara, K., H. Hohjoh, T. Inazumi, S. Tsuchiya, and Y. Sugimoto (2015) Prostaglandin E2-induced inflammation: relevance of prostaglandin E receptors. Biochim Biophys Acta. 1851: 414–421.
Süleyman, H., B. Demircan, and Y. Karagöz (2007) Antiinflammatory and side effects of cyclooxygenase inhibitors. Pharmacol. Rep. 59: 247–258.
Otsuka, S., T. Aoyama, M. Furu, K. Ito, Y. Jin, A. Nasu, K. Fukiage, Y. Kohno, T. Maruyama, T. Kanajik, A. Nishiura, H. Sugiharak, S. Fujimurak, T. Otsuka, T. Nakamura, and J. Toguchida (2009) PGE2 signal via EP2 receptors evoked by a selective agonist enhances regeneration of injured articular cartilage. Osteoarthritis Cartilage. 17: 529–538.
Chizzolini, C. and N. C. Brembilla (2009) Prostaglandin E2: igniting the fire. Immunol. Cell Biol. 87: 510–511.
Yokoyama, U., K. Iwatsubo, M. Umemura, T. Fujita, and Y. Ishikawa (2013) The prostanoid EP4 receptor and its signaling pathway. Pharmacol. Rev. 65: 1010–1052.
Ensor, C. M. and H. H. Tai (1994) Bacterial expression and sitedirected mutagenesis of two critical residues (tyrosine-151 and lysine-155) of human placental NAD+-dependent 15-hydroxyprostaglandin dehydrogenase. Biochim Biophys Acta. 1208: 151–156.
Hamza, A., H. Cho, H. H. Tai, and C. G. Zhan (2005) Understanding human 15-hydroxyprostaglandin dehydrogenase binding with NAD+ and PGE2 by homology modeling, docking and molecular dynamics simulation. Bioorg. Med. Chem. 13: 4544–4551.
Cho, H., L. Huang, A. Hamza, D. Gao, C. G. Zhan, and H. H. Tai (2006) Role of glutamine 148 of human 15-hydroxyprostaglandin dehydrogenase in catalytic oxidation of prostaglandin E2. Bioorg. Med. Chem. 14: 6486–6491.
Lee, J. S., K. R. Lee, S. Lee, H. J. Lee, H. S. Yang, J. Yeo, J. M. Park, B. H. Choi, and E. K. Hong (2017) Polysaccharides isolated from liquid culture broth of Inonotusobliquus inhibit the invasion of human non-small cell Lung carcinoma cells. Biotechnol. Bioprocess Eng. 22: 45–51.
Oh, M., Y. J. Kim, Y. J. Son, H. S. Yoo, and J. H. Park (2017) Promotive effects of human induced pluripotent stem cellconditioned medium on the proliferation and migration of dermal fibroblasts. Biotechnol. Bioprocess Eng. 22: 561–568.
Boukamp, P., R. T. Petrussevska, D. Breitkreutz, J. Hornung, A. Markham, and N. E. Fusenig (1988) Normal keratinization in a spontaneously immortalized Aneuploidhuman keratinocyte cell line. J. Cell Bio. 106: 761–771.
Kaur Manjal, S., R. Kaur, R. Bhatia, K. Kumar, V. Singh, R. Shankar, R. Kaur, and R. K. Rawal (2017) Synthetic and medicinal perspective of thiazolidinones. Bioorg Chem. 75: 406–423.
Verma, A. and S. K. Saraf (2008) 4-thiazolidinone—a biologically active scaffold. Eur. J. Med. Chem. 43: 897–905.
Jo, D. R., Y. O. Kim, R. Kim, Y. C. Chang, D. B. Choi, and H. Cho (2017) Novel rhodanine derivatives are selective algicides against Microcystis aeruginosa. Biotechnol. Bioprocess Eng. 22: 748–757.
You, D. S., Y. W. Lee, D. B. Choi, Y. C. Chang, and H. Cho (2017) Algicidal effects of thiazolinedione derivatives against Microcystis aeruginosa. Korean J. Chem. Eng. 34: 139–149.
Cho, H. and H. H. Tai (2002) Thiazolidinediones as a novel class of NAD (+)-dependent 15-hydroxyprostaglandin dehydrogenase inhibitors. Arch. Biochem. Biophys. 405: 247–251.
Wu, Y., H. H. Tai, and H. Cho (2010) Synthesis and SAR of thiazolidinedione derivatives as 15-PGDH inhibitors. Bioorg. Med. Chem. 18: 1428–1433.
Wu, Y., S. Karna, C. H. Choi, M. Tong, H. H. Tai, D. H. Na, C. H. Jang, and H. Cho (2011) Synthesis and biological evaluation of novel thiazolidinedione analogues as 15-hydroxyprostaglandindehydrogenase inhibitors. J. Med. Chem. 54: 5260–5264.
Choi, D. B., Y. L. Piao, Y. Wu, and H. Cho (2013) Control of the intracellular levels of prostaglandin E2 through inhibition of the 15-hydroxyprostaglandin dehydrogenase for wound healing. Bioorg. Med. Chem. 21: 4477–4484.
Clark, R. A. (1996) Wound repair: Overview and general considerations. pp. 3–50. In: R. A. Clark ( ed. ). The Molecular and Cellular Biology of Wound Repair. Springer US, New York, USA.
Werner, S. and R. Grose (2003) Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 83: 835–870.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2016R1D1A1B04930255).
The authors declare no conflict of interest.
Neither ethical approval nor informed consent was required for this study.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Na, A.R., Choi, D. & Cho, H. Synthesis, Structural Analysis, and Biological Evaluation of Novel ((2,4-dioxothiazolidin-5-ylidene)methyl)phenyl Derivatives. Biotechnol Bioproc E 25, 149–163 (2020). https://doi.org/10.1007/s12257-019-0308-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-019-0308-y