Abstract
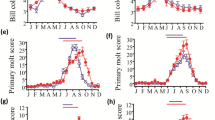
Photoperiodism has been shown to be an important synchronizer of seasonal reproduction in many rodent species in the wild; it is a reliable cue as in the southern hemisphere it coincides with the onset of rainfall and hence the availability of food resources for maximal reproductive success. The photoperiodic effect on the reproductive status of the Namibian gerbil, Gerbilliscus cf. leucogaster from central Namibia was investigated. Twenty adult males were exposed to a long-day length (16L:8D), while further 20 adult males were subjected to a short-day length (SD:8L:16D); all for a period of 3 months. Testicular mass per gram body mass, testicular volume and seminiferous tubule diameters were used to assess the effect of photoperiod on gonadal development. Body mass did not significantly differ between the two photoperiodic regimes. The testicular mass per gram of body mass was significantly heavier for the males maintained on a long photoperiod compared to those on a short photoperiod. Similarly, testicular volume and seminiferous tubule diameter were greater in males maintained on a long-day cycle compared to those on the short-day cycle. These findings suggest that G. cf. leucogaster is photoresponsive to day length changes. Photoperiodic changes in the semi-arid habitats can be used to herald the onset of reproduction as it often acts in concert with other proximate cues in desert rodents, but is a constant environmental cue that does not change from year to year, unlike rainfall patterns.

Similar content being viewed by others
References
Alibhai SK (1986) Reproductive response of Gerbillus harwoodii to 6-MBOA in the Kora National Reserve, Kenya. J Trop Pediatr 2:377–379
Anand S, Losee-Olson S, Turek FW, Horton TH (2002) Differential regulation of luteinizing hormone and follicle-stimulating hormone in male Siberian hamster by exposure to females and photoperiod. Endocrinology 143:2178–2188
Bartness TJ, Goldman BD (1989) Mammalian pineal melatonin: a clock for all seasons. Experientia 45:939–945
Bartness TJ, Wade GN (1985) Seasonal body weight cycles in hamsters. Neurosci Biobehav Rev 9:599–612
Bastos ADS, Nair D, Taylor PJ, Brettschneider H, Kirsten F, Mostert ME, von Maltitz E, Lamb JM, van Hooft P, Belmain SR, Contrafatto G, Downs S, Chimimba CT (2011) Genetic monitoring detects an overlooked cryptic species and reveals the diversity and distribution of three invasive Rattus congeners in South Africa. BMC Genet 12:26
Benson GK, Morris LR (1971) Foetal growth and lactation in rats exposed to high temperatures during pregnancy. J Reprod Fertil 27:369–384
Ben-Zaken I, Haim A, Zubidat AE (2013) Long-day photoperiod interacts with vasopressin and food restriction to modulate reproductive status and vasopressin receptor expression of male golden spiny mice. J Exp Biol 216:3495–3503. https://doi.org/10.1242/jeb.089607
Bernard RTF, Hall J (1995) Failure of the oestrous cycle and spermatogenesis to respond to day length in subtropical African rodent, the pouched mouse (Saccostomus campestris). Biol Reprod 52:1291–1296
Bronson FH (1985) Mammalian reproduction: an ecological perspective. Biol Reprod 32:1–26
Bronson FH (1989) Regulation by social cues. In: Bronson FH (ed) Mammalian reproductive biology. University of Chicago Press, Chicago
Bronson FH (2009) Climate change and seasonal reproduction in mammals. Philos Trans R Soc Lond Biol 364:3331–3340
Bronson FH, Prayor S (1983) Ambient temperature and reproductive success in rodent living at different latitudes. Biol Reprod 29:72–80
Bukreeva OM, Lidzhi-Garyaeva GV (2018) Mass migration of social voles (Microtus socialis Pallas, 1773) in the Northwestern Caspian Region. Arid Ecosyst 8:147–151. https://doi.org/10.1134/S2079096118020038
Duncan MJ, Goldman BD (1984) Hormonal regulation of the annual pelage color cycle in the Djungarian hamster, Phodopus sungorus. II. Role of prolactin. J Exp Zool 230:97–103
El-Bakry HA, Zaharan WM, Bartness TJ (1998) Photoperiod responses of four wild trapped desert rodent species. Am J Physiol 275:2012–2022
Fabio-Braga AP, Klein W (2018) Temperature and circadian effects on metabolic rate of South American echimyid rodents, Trinomys setosus and Clyomys bishopi (Rodentia: Echimyidae). Zoologia 35:1–6
Flowerdew JR (1987) Effects of the environment on reproduction. In: Flowerdew JR (ed) Mammals, their reproductive biology and population ecology. Edward Arnold Publishers Ltd, London
Forger NG, Zucker I (1985) Photoperiodic regulation of reproductive development in male white-footed mice (Peromyscus leucopus) born at different phases of breeding season. J Reprod Fertil 73:271–278
Frost D, Zucker I (1983) Photoperiodic and melatonin influences seasonal gonadal cycles in the grasshopper mouse (Onychomys leucogaster). J Reprod Fertil 69:237–244
The Goddard Institute for Space Studies (1957–1988) Columbia University, New York City, USA
Hamid HY, Zakaria MZAB, Meng GY Haron, Abd W, Mustapha NM (2012) Effects of elevated ambient temperature on reproductive outcomes and offspring growth depend on exposure time. Sci World J 2012:1–6. https://doi.org/10.1100/2012/359134
Heideman PD, Sylvester CJ (1997) Reproductive photo responsiveness in unmanipulated male Fischer 344 laboratory rats. J Reprod 57(1):134–138
Heidemann PD, Deiler RW, York M (1998) Food and neonatal androgen interact with photoperiod to inhibit reproductive maturation in Fischer 344 rats. Biol Reprod 59:358–363
Heldmaier G, Steinlechner S, Rafael J, Vsiansky P (1981) Photoperiodic control and effects of melatonin on non-shivering thermogenesis and brown adipose tissue. Science 212:917–919
Hoffmann K (1973) The infl uence of photoperiod and melatonin on testis size, body weight, and pelage colour in the Djungarian hamster (Phodopus sungorus). J Comput Physiol A 85:267–282
Ims RA (1990) The ecology and evolution of reproductive synchrony. Trends Ecol Evol 5:135–140
Jackson C, Bernard RTF (1999) Short day length alone does not inhibit spermatogenesis in the seasonally breeding four-stripped field mouse (Rhabdomys pumilio). Biol Reprod 60:1320–1323
Jameson EW (1988) The patterns of seasonality, Ch. 13. In: Jameson EW (ed) Vertebrate reproduction. A Wiley Interscience publication. Wiley, New York
Kenagy J, Bartholomew A (1981) Effects of day length, temperature and green food on testicular development in a dessert mouse, Perognathus formosus. Physiol Zool 54:62–73
Kenagy GJ, Bartholomew GA (1985) Seasonal reproductive patterns in five coexisting California desert rodent species. Ecol Monogr 55:371–397
Kerbeshian MC, Bronson FH (1996) Running-induced testicular recrudescence in the meadow vole: role of the circadian system. Physiol Behav 60:165–170
Kerbeshian MC, Bronson FH, Bellis ED (1994) Variation in reproductive photo responsiveness in a wild population of meadow voles. Biol Reprod 50:7445–7450
Kriegsfeld LJ, Drazen DL, Nelson RJ (1999) Effects of photoperiod and reproductive responsiveness on pituitary sensitivity to GnRH in male prairie voles (Microtus ochrogaster). Gen Comp Endocrinol 116(2):221–228
Leeson CR, Leeson TS, Paparo AA (1985) Textbook of histology. WB Saunders Company, Philadelphia
McCullagh P, Nelda J (1989) General linear models, 2nd edn. Chapman and Hall, Boca Raton
McDonald JH (2014a) Using spreadsheets for statistics. In: McDonald JH (ed) Handbook of biological statistics. Sparky House Publishing, Baltimore, pp 266–273
McDonald JH (2014b) Getting started with SAS. In: McDonald JH (ed) Handbook of biological statistics. Sparky House Publishing, Baltimore, pp 285–292
Meheretu Y, Welegerima K, Sluydts V, Bauer H, Gebrehiwot K, Deckers J, Makundi R, Leirs H (2015) Reproduction and survival of rodents in crop fields: the effects of rainfall, crop stage and stone-bund density. Wildl Res 42(2):158–164. https://doi.org/10.1071/WR14121
Muteka SP, Chimimba CT, Bennett NC (2006) Reproductive photoresponsiveness in Aethomys ineptus and A. namaquensis (Rodentia: Muridae) from southern Africa. J Zool Lond 268:225–231
Mutze G (2007) Does high growth rate of juvenile house mice with prolonged access to ripening grain and water drive population outbreaks? N Z J Zool 4:195–202
Namibia Meteorological Service (2014) 12C Hugel Street, Windhoek, Namibia
Nelson RJ, Dark J, Zucker I (1983) Influence of photoperiod, nutrition and water availability on the reproduction of male California vole (Microtus californicus). J Reprod Fertil 69:473–477
Nelson RJ, Kita M, Blom MC, Rhyme-Grey J (1992) Photoperiod influences the critical caloric intake necessary to maintain reproduction among male deer mice (Peromyscus maniculatus). Biol Reprod 46:226–232
Nilsson P (2001) The biological triangle of life. Interaction between nutrition, reproduction and longevity. Lakartidningen 98:1797–1800
Prendergast BJ, Kriegsfeld LJ, Nelson RJ (2001) Photoperiodic polymorphism in rodents: neuroendocrine mechanisms, costs, and functions. Q Rev Biol 76:293–325
Prendergast BJ, Zucker I, Nelson RJ (2009) Seasonal rhythms of mammalian behavioral neuroendocrinology. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Moss R, Rubin R (eds) Hormones, brain, and behavior, vol 1, 2nd edn. Academic Press, San Diego
Rani S, Kumar V (2014) Photoperiodic regulation of seasonal reproduction in higher vertebrates. Indian J Exp Biol 52:413–419
Ross MH, Romrell LJ, Kaye GI (1995) Histology: text and atlas. William & Wilkins, Baltimore
Sangeeta R, Vinod K (2014) Photoperiodic regulation of seasonal reproduction in higher vertebrates. Indian J Exp Biol 52(5):413–419
Scheffer TH (1924) Notes on the breeding of Peromyscus. J Mammal 5:258–260
Schlatt S, Niklowitz P, Hoffmann K, Nieschlag E (1993) Influence of short photoperiods on reproductive organs and estrous cycles of normal and pinealectomized female Djungarian hamsters, Phodopus sungorus. Biol Reprod 49:243–250
Sicard B, Fuminier F, Maurel D, Boissin J (1993) Temperature and water conditions mediate the effects of day length on the breeding cycle of a Sahelian rodent, Arvicanthis niloticus. Biol Reprod 49:716–722
Spears N, Clarke JR (1986) Effects of male presence and of photoperiod on the sexual maturation of the field vole (Microtus agrestis). J Reprod Fertil 78:231–238
Tavolaro FM, Thomson LM, Ross AW, Morgan PJ, Helfer G (2015) Photoperiodic effects on seasonal physiology, reproductive status and hypothalamic gene expression in young male F344 rats. J Neuroendocrinol 27:79–87
Tinney GM, Bernard RTF, White RM (2001) Influences of food quality and quantity on the male reproductive organs of a seasonally breeding rodent, the pouched mouse (Saccostomus campestris), from a seasonal but unpredictable environment. Afr Zool 36:23–30
Wade GN, Bartness TJ (1984) Effects of photoperiod and gonadectomy on food intake, body weight, and body composition in Siberian hamsters. Am J Physiol 246:R26–R30
Whitestt JM, Miller LL (1982) Photoperiod and reproduction in female deer mice. Biol Reprod 26:296–304
Woodall PF, Skinner JD (1989) Seasonality of reproduction in male rock shrews, Elephantulus myurus. J Zool Lond 217:203–212
Young KA, Zirkin BR, Nelson RJ (2000) Testicular regression in response to food restriction and short photoperiod in white-footed mice (Peromyscus leucopus) is mediated by Apoptosis. Biol Reprod 62:347–354
Yu H-S, Reiter RJ, Goldman BD, Nelson RJ (1993) Melatonin and seasonality in mammals. In: Yu H-S, Reiter RJ (eds) Melatonin—biosynthesis, physiological effects, and clinical applications. CRC Press, Boca Raton, pp 225–252
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling editor: Sabine Begall.
Rights and permissions
About this article
Cite this article
Muteka, S.P., Chimimba, C.T., Bastos, A.D.S. et al. Photoperiodic effects on the male gonads of the Namibian gerbil, Gerbilliscus cf. leucogaster from central Namibia. Mamm Biol 100, 165–171 (2020). https://doi.org/10.1007/s42991-020-00008-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42991-020-00008-y