Abstract
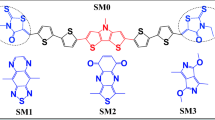
The end groups of small-molecule acceptors strongly affect their properties and performance. In this study, theoretical analysis is performed to determine the reason why two similar molecules differing in their end group show very different performance in organic solar cells. The 1,1-dicyanomethylene-3-indanone-based small-molecule acceptor (DC-IDT2Tz) shows a higher transition dipole moment as compared with the rhodanine-based small acceptor (RD-IDT2Tz). DC-IDT2Tz presents a lower exciton binding energy as compared with RD-IDT2Tz. Moreover, DC-IDT2Tz shows a lower reorganization energy and higher transfer integrals than RD-IDT2Tz. All these theoretical descriptors can explain the reason behind the higher efficiency of DC-IDT2Tz. The studied theoretical parameters can help to verify the performance of organic semiconductors before synthesis. This work will thus be helpful for experimental scientists to screen compounds and select the best one for synthesis.
Graphic abstract











Similar content being viewed by others
References
Mahmood, A., et al.: Recent progress in porphyrin-based materials for organic solar cells. J. Mater. Chem. A 6(35), 16769–16797 (2018)
Tang, A., et al.: Design of diketopyrrolopyrrole (DPP)-based small molecules for organic-solar-cell applications. Adv. Mater. 29(2), 1600013 (2017)
Du, M., et al.: Wide-band-gap phthalimide-based D-π-A polymers for nonfullerene organic solar cells: the effect of conjugated π-bridge from thiophene to thieno[3,2-b]thiophene. J. Phys. Chem. C 124(1), 230–236 (2020)
Yao, H., et al.: Molecular design of benzodithiophene-based organic photovoltaic materials. Chem. Rev. 116(12), 7397–7457 (2016)
Lin, Y., Zhan, X.: Oligomer molecules for efficient organic photovoltaics. Acc. Chem. Res. 49(2), 175–183 (2016)
Lin, Y., Li, Y., Zhan, X.: Small molecule semiconductors for high-efficiency organic photovoltaics. Chem. Soc. Rev. 41(11), 4245–4272 (2012)
Mohajeri, A., Omidvar, A.: Fullerene-based materials for solar cell applications: design of novel acceptors for efficient polymer solar cells—a DFT study. Phys. Chem. Chem. Phys. 17(34), 22367–22376 (2015)
He, Y., Li, Y.: Fullerene derivative acceptors for high performance polymer solar cells. Phys. Chem. Chem. Phys. 13(6), 1970–1983 (2011)
Li, C.-Z., Yip, H.-L., Jen, A.K.Y.: Functional fullerenes for organic photovoltaics. J. Mater. Chem. 22(10), 4161–4177 (2012)
He, D., et al.: A highly efficient fullerene acceptor for polymer solar cells. Phys. Chem. Chem. Phys. 16(16), 7205–7208 (2014)
Mahmood, A., et al.: A novel thiazole based acceptor for fullerene-free organic solar cells. Dyes Pigm. 149, 470–474 (2018)
Mahmood, A., et al.: Introducing four 1,1-dicyanomethylene-3-indanone end-capped groups as an alternative strategy for the design of small-molecular nonfullerene acceptors. J. Phys. Chem. C 122(51), 29122–29128 (2018)
Xiao, B., et al.: A comparison of n-type copolymers based on cyclopentadithiophene and naphthalene diimide/perylene diimides for all-polymer solar cell applications. Polym. Chem. 6(43), 7594–7602 (2015)
Xiao, B., et al.: Quinoxaline-containing nonfullerene small-molecule acceptors with a linear A2-A1-D-A1-A2 skeleton for poly(3-hexylthiophene)-based organic solar cells. ACS Appl. Mater. Interfaces 10(12), 10254–10261 (2018)
Xiao, B., et al.: Achievement of high Voc of 1.02 V for P3HT-based organic solar cell using a benzotriazole-containing non-fullerene acceptor. Adv. Energy Mater. 7(8), 1602269 (2017)
Li, J., et al.: A thieno[3,4-b]pyrazine-based A2–A1–D–A1–A2 type low bandgap non-fullerene acceptor with 1,1-dicyanomethylene-3-indanone (IC) as the terminal group. J. Mater. Chem. C 7(29), 8820–8824 (2019)
Tang, A., et al.: Benzotriazole-based acceptor and donors, coupled with chlorination, achieve a high Voc of 1.24 V and an efficiency of 10.5% in fullerene-free organic solar cells. Chem. Mater. 31(11), 3941–3947 (2019)
Xiao, B., et al.: Effects of oxygen atoms introduced at different positions of non-fullerene acceptors in the performance of organic solar cells with poly(3-hexylthiophene). ACS Appl. Mater. Interfaces 12(1), 1094–1102 (2020)
Chen, Y., et al.: Benzotriazole-based p-type polymers with thieno[3,2-b]thiophene π-bridges and fluorine substituents to realize high Voc. ACS Appl. Polym. Mater. 1(4), 906–913 (2019)
Li, J., et al.: The first thieno[3,4-b]pyrazine based small molecular acceptor with a linear A2–A1–D–A1–A2 skeleton for fullerene-free organic solar cells with a high Voc of 1.05 V. Chem. Commun. 54(76), 10770–10773 (2018)
Chen, Y., et al.: Changing the π-bridge from thiophene to thieno[3,2-b]thiophene for the D–π–A type polymer enables high performance fullerene-free organic solar cells. Chem. Commun. 55(47), 6708–6710 (2019)
Hu, Y., et al.: Theoretical investigation on the crystal structures and electron transfer properties of cyanated TTPO and their selenium analogs. J. Mater. Sci. 51(13), 6235–6248 (2016)
Zhu, L., et al.: Achieving small exciton binding energies in small molecule acceptors for organic solar cells: effect of molecular packing. J. Phys. Chem. Lett. 10(17), 4888–4894 (2019)
Lu, X., et al.: Effect of the functionalized π-bridge on porphyrin sensitizers for dye-sensitized solar cells: an in-depth analysis of electronic structure, spectrum, excitation, and intramolecular electron transfer. J. Mater. Chem. C 3(39), 10129–10139 (2015)
Sancho-García, J.C.: Assessment of density-functional models for organic molecular semiconductors: the role of Hartree-Fock exchange in charge-transfer processes. Chem. Phys. 331(2), 321–331 (2007)
Liu, X., Liu, Y., Zheng, Y.: Charge transfer mobility of naphthodithiophenediimide derivative: normal-mode and bond length relaxation analysis. Chem. Phys. Lett. 645, 92–96 (2016)
Touhami, A., Chaabane, R.B., Allouche, A.R.: Theoretical investigation on electronic, optical, and charge transport properties of new anthracene derivatives. Comput. Theor. Chem. 1073, 123–130 (2015)
Wen, S.-H., et al.: First-principles investigation of anistropic hole mobilities in organic semiconductors. J. Phys. Chem. B 113(26), 8813–8819 (2009)
Irfan, A., et al.: Exploration the effect of metal and electron withdrawing groups on charge transport and optoelectronic nature of schiff base Ni(II), Cu(II) and Zn(II) complexes at molecular and solid-state bulk scales. Mater. Sci. Semicond. Process. 107, 104855 (2020)
Irfan, A.: Exploring the effect of oligocene elongation on photovoltaic, optoelectronic and charge transfer properties in TPA dyes tethered to the semiconductor surface. Res. Phys. 13, 102304 (2019)
Irfan, A.: Comparison of mono- and di-substituted triphenylamine and carbazole based sensitizers @(TiO2)38 cluster for dye-sensitized solar cells applications. Comput. Theor. Chem. 1159, 1–6 (2019)
Cao, F.-Y., et al.: Forced coplanarity of dithienofluorene-based non-fullerene acceptors to achieve high-efficiency organic solar cells. J. Mater. Chem. A 7(30), 17947–17953 (2019)
Li, M., et al.: Tuning the dipole moments of nonfullerene acceptors with an asymmetric terminal strategy for highly efficient organic solar cells. J. Mater. Chem. A 7(15), 8889–8896 (2019)
Tian, H., et al.: A triphenylamine dye model for the study of intramolecular energy transfer and charge transfer in dye-sensitized solar cells. Adv. Funct. Mater. 18(21), 3461–3468 (2008)
Lincker, F., et al.: Fluorenone-based molecules for bulk-heterojunction solar cells: synthesis, characterization, and photovoltaic properties. Adv. Funct. Mater. 18(21), 3444–3453 (2008)
Cho, N., et al.: Synthesis and characterization of push–pull organic semiconductors with various acceptors for solution-processed small molecule organic solar cells. Tetrahedron 68(21), 4029–4036 (2012)
Yassar, A., Videlot, C., Jaafari, A.: Synthesis and photovoltaic properties of mono-substituted quaterthiophenes bearing strong electron-withdrawing group. Sol. Energy Mater. Sol. Cells 90(7), 916–922 (2006)
Li, Y., et al.: Novel low-bandgap oligothiophene-based donor–acceptor alternating conjugated copolymers: synthesis, properties, and photovoltaic applications. J. Polym. Sci. Part A: Polym. Chem. 48(13), 2765–2776 (2010)
Li, W., et al.: D-A-π-A featured sensitizers bearing phthalimide and benzotriazole as auxiliary acceptor: effect on absorption and charge recombination dynamics in dye-sensitized solar cells. ACS Appl. Mater. Interfaces 4(3), 1822–1830 (2012)
Li, Z., et al.: Design and synthesis of solution processable small molecules towards high photovoltaic performance. J. Mater. Chem. 21(7), 2159–2168 (2011)
Pai, C.-L., et al.: Electronic structure and properties of alternating donor–acceptor conjugated copolymers: 3,4-Ethylenedioxythiophene (EDOT) copolymers and model compounds. Polymer 47(2), 699–708 (2006)
Skabara, P.J., et al.: Fluorene functionalised sexithiophenes—utilising intramolecular charge transfer to extend the photocurrent spectrum in organic solar cells. J. Mater. Chem. 17(11), 1055–1062 (2007)
Lee, J.Y., et al.: Synthesis of random copolymers based on 3-hexylthiophene and quinoxaline derivative: influence between the intramolecular charge transfer (ICT) effect and π-conjugation length for their photovoltaic properties. Synth. Met. 161(1), 1–6 (2011)
Jung, I.H., et al.: Synthesis and characterization of fluorene and cyclopentadithiophene-based copolymers exhibiting broad absorption for photovoltaic devices. J. Polym. Sci. Part A: Polym. Chem. 49(5), 1248–1255 (2011)
Kim, Y.-G., et al.: Variable band gap conjugated polymers for optoelectronic and redox applications. J. Mater. Res. 20(12), 3188–3198 (2005)
Cheng, Y.-J., et al.: Alternating copolymers incorporating cyclopenta[2,1-b:3,4-b′]dithiophene unit and organic dyes for photovoltaic applications. J. Polym. Sci. Part A: Polym. Chem. 49(8), 1791–1801 (2011)
Li, Y., et al.: Molecular structure–property engineering for photovoltaic applications: fluorene-acceptor alternating conjugated copolymers with varied bridged moieties. Polymer 51(8), 1786–1795 (2010)
Jung, I.H., et al.: Synthesis and photovoltaic properties of cyclopentadithiophene-based low-bandgap copolymers that contain electron-withdrawing thiazole derivatives. Chem. A Eur. J. 16(12), 3743–3752 (2010)
Ranjith, K., et al.: Dithienylcyclopentadienone derivative-co-benzothiadiazole: an alternating copolymer for organic photovoltaics. Sol. Energy Mater. Sol. Cells 98, 448–454 (2012)
Li, Y., et al.: Energy level and molecular structure engineering of conjugated donor–acceptor copolymers for photovoltaic applications. Macromolecules 42(13), 4491–4499 (2009)
Han, G., et al.: Terminal π–π stacking determines three-dimensional molecular packing and isotropic charge transport in an A–π–A electron acceptor for non-fullerene organic solar cells. J. Mater. Chem. C 5(20), 4852–4857 (2017)
Mai, J., et al.: Hidden structure ordering along backbone of fused-ring electron acceptors enhanced by ternary bulk heterojunction. Adv. Mater. 30(34), 1802888 (2018)
Li, C., et al.: A nonfullerene acceptor utilizing a novel asymmetric multifused-ring core unit for highly efficient organic solar cells. J. Mater. Chem. C 6(18), 4873–4877 (2018)
Yin, S., et al.: Balanced carrier transports of electrons and holes in silole-based compounds—a theoretical study. J. Phys. Chem. A 110(22), 7138–7143 (2006)
Troisi, A., Orlandi, G.: The hole transfer in DNA: calculation of electron coupling between close bases. Chem. Phys. Lett. 344(5), 509–518 (2001)
Sutter, A., et al.: Photovoltaic performance of novel push–pull–push thienyl–Bodipy dyes in solution-processed BHJ-solar cells. New J. Chem. 38(4), 1701–1710 (2014)
Kawabata, K., et al.: Very small bandgap π-conjugated polymers with extended thienoquinoids. J. Am. Chem. Soc. 138(24), 7725–7732 (2016)
Sancho-García, J.C., Moral, M., Pérez-Jiménez, A.J.: Effect of cyclic topology on charge-transfer properties of organic molecular semiconductors: the case of cycloparaphenylene molecules. J. Phys. Chem. C 120(17), 9104–9111 (2016)
Calvo-Castro, J., et al.: Intermolecular interactions and energetics in the crystalline π–π stacks and associated model dimer systems of asymmetric halogenated diketopyrrolopyrroles. Cryst. Growth Des. 16(3), 1531–1542 (2016)
Acknowledgements
The authors thank the Deanship of Scientific Research at King Khalid University for funding this work through research groups program under grant no. R.G.P.2/44/40. A.M. sincerely acknowledge the CAS-TWAS President‘s Fellowship Program for providing financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mahmood, A., Irfan, A. Computational analysis to understand the performance difference between two small-molecule acceptors differing in their terminal electron-deficient group. J Comput Electron 19, 931–939 (2020). https://doi.org/10.1007/s10825-020-01494-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10825-020-01494-6