Abstract
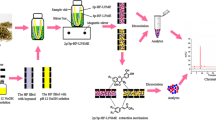
A three-phase hollow fiber liquid-phase microextraction based on reversed micellar supramolecule was developed and employed for the determination of quality markers of three anthraquinones in Rhubarb and their plasma protein binding rates. Moreover, the extraction mechanism of reversed micellar supramolecule and determination mechanism of plasma protein binding rate by the proposed method are both explored. In this procedure, n-nonanol was impregnated in the wall pores and reverse micelle of tetrabutylammonium chloride/n-nonanol filled in lumen of hollow fibers, which were covered with a thin salt film and used for the microextraction of target compounds prior to HPLC analysis. Important extraction parameters including extraction solvent type, reversed micellar supramolecule type and concentration, sample phase pH, salt concentration, stirring rate, extraction time, and binding time between active compound and plasma protein were investigated. Under the optimized conditions, limits of detection were 0.1–0.4 ng/mL with enrichment factors in the range of 54.2–100.7. The linearities were 0.5–500 ng/mL with r2 ≥ 0.9838. Satisfactory accuracies (relative recoveries 95.7–98.3%) were also obtained. The average plasma protein binding rates of rhein, chrysophenol, and physcion were 96.7%, 41.8%, and 46.7%, respectively. The results showed that the proposed procedure can not only extract and concentrate anthraquinones in Rhubarb, but also determine their plasma protein binding rates.
Graphic Abstract





Similar content being viewed by others
Abbreviations
- ACTPPBR :
-
Active compound-total plasma protein binding rate
- EE :
-
Extraction efficiency
- EF :
-
Enrichment factor
- HF:
-
Hollow fiber
- HF-LPME:
-
Hollow fiber liquid-phase microextraction
- HPC:
-
Hexadecylpyridinium chloride monohydrate
- LPME:
-
Liquid-phase microextraction
- MTAC:
-
Methyltrioctylammonium chloride
- Q-marker:
-
Quality marker
- RM-HF-LPME:
-
Reverse micelle hollow fiber liquid-phase microextraction
- RMS:
-
Reversed micellar supramolecule
- TBAC:
-
Tetrabutylammonium chloride
- TCM:
-
Traditional Chinese medicine
References
Qin Y, Wang JB, Kong WJ, Zhao YL, Yang HY, Dai CM, Fang F, Zhang L, Li BC, Jin C, Xiao XH (2011) The diarrhoeogenic and antidiarrhoeal bidirectional effects of rhubarb and its potential mechanism. J Ethnopharmacol 133:1096–1102
Tseng SH, Lee HH, Chen LG, Wu CH, Wang CC (2006) Effects of three purgative decoctions on inflammatory mediators. J Ethnopharmacol 105:118–124
Zhao YL, Wang JB, Zhou GD, Shan LM, Xiao XH (2010) Investigations of free anthraquinones from Rhubarb against α-Naphthylisothiocyanate-induced cholestatic liver injury in rats. Basic Clin Pharmacol Toxicol 104:463–469
He ZH, He MF, Ma SC, But PH (2009) Anti-angiogenic effects of rhubarb and its anthraquinone derivatives. J Ethnopharmacol 121:313–317
Singh NP, Gupta AP, Sinha AK, Ahuja PS (2005) High-performance thin layer chromatography method for quantitative determination of four major anthraquinone derivatives in Rheum emodi. J Chromatogr A 1077:202–206
Liu R, Li A, Sun A (2004) Preparative isolation and purification of hydroxyanthraquinones and cinnamic acid from the Chinese medicinal herb Rheum officinale Baill. by high-speed counter-current chromatography. J Chromatogr A 1052:217–221
Lin ZJ, Musiano D, Abbot A, Shum L (2005) In vitro plasma protein binding determination of flunarizine using equilibrium dialysis and liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal 37:757–762
Ian De Veau EJ (1999) Determination of non-protein bound phenylbutazone in bovine plasma using ultrafiltration and liquid chromatography with ultraviolet detection. J Chromatogr B Biomed Sci Appl 721:141–145
Verbeeck RK (2000) Blood microdialysis in pharmacokinetic and drug metabolism studies. Adv Drug Deliv Rev 45:217–228
Shibukawa A, Ishizawa N, Kimura T, Sakamoto Y, Ogita K, Matsuo Y, Kuroda Y, Matayatsuk C, Nakagawa T, Wainer IW (2002) Plasma protein binding study of oxybutynin by high-performance frontal analysis. J Chromatogr B 768:177–188
Li J, Shi Q, Jiang Y, Liu Y (2015) Pretreatment of plasma samples by a novel hollow fiber centrifugal ultrafiltration technique for the determination of plasma protein binding of three coumarins using acetone as protein binding releasing agent. J Chromatogr B 1001:114–123
Liang JW, Hsiu SL, Wu PP, Chao PDL (1995) Emodin pharmacokinetics in rabbits. Planta Med 61:406–408
Pedersen-Bjergaard S, Rasmussen KE (1999) Liquid-liquid-liquid microextraction for sample preparation of biological fluids prior to capillary electrophoresis. Anal Chem 71:2650–2656
Li MM, Hu S, Chen X, Bai XH (2017) Development of a novel hollow-fiber liquid-phase microextraction based on oil-in-salt and its comparison with conventional one. J Sep Sci 40:2941–2949
Xue J, Wang RQ, Chen X, Hu S, Bai XH (2019) Three-phase hollow-fiber liquid-phase microextraction based on deep eutectic solvent as acceptor phase for extraction and preconcentration of main active compounds in a traditional Chinese medicinal formula. J Sep Sci 42:2239–2246
Hatami M, Farhadi K (2012) Application of hollow fiber-supported liquid-phase microextraction coupled with HPLC for the determination of guaifenesin enantiomer-protein binding. Biomed Chromatogr 26:875–880
Li MM, Chen X, Hu S, Wang RQ, Peng XL, Bai XH (2018) Determination of blood concentrations of main active compounds in Zi-Cao-Cheng-Qi decoction and their total plasma protein binding rates based on hollow fiber liquid phase microextraction coupled with high performance liquid chromatography. J Chromatogr B 1072:355–361
Stano P, Luisi PL (2008) Self-reproduction of micelles, reverse micelles, and vesicles. Adv Planar Lipid Bilayers Liposomes 7:221–263
Banforuzi SR, Hadjmohammadi MR (2017) Two-phase hollow fiber-liquid liquid microextraction based on reverse micelle for the determination of quercetin in human plasma and vegetables samples. Talanta 173:14–21
Banforuzi SR, Hadjmohammadi MR (2018) Application of non-ionic surfactant as a developed method for the enhancement of two-phase solvent bar microextraction for the simultaneous determination of three phthalate esters from water samples. J Chromatogr A 1561:39–47
Banforuzi SR, Hadjmohammadi MR (2017) Solvent bar microextraction using a reverse micelle containing extraction phase for the determination of warfarin from human plasma by high-performance liquid chromatography. J Chromatogr A 1496:1–8
Liu ZC, Zhang SM, Wang RQ, Chen X, Hu S, Bai XH (2019) Comparison of three phase hollow fiber liquid-phase microextraction based on reverse micelle with conventional two phase hollow fiber liquid-phase microextraction and their applications for analysis of cinnamic acids in traditional Chinese medicines. J Sep Sci 42:2977–2984
Wang Z, Hu J, Du H, He S, Li Q, Zhang H (2016) Microwave-assisted ionic liquid homogeneous liquid-liquid microextraction coupled with high performance liquid chromatography for the determination of anthraquinones in Rheum palmatum L. J Pharm Biomed Anal 125:178–185
Hu SS, Cao W, Dai HB, Da JH, Ye LH, Cao J, Li XY (2014) Ionic-liquid-micelle-functionalized mesoporous Fe3O4 microspheres for ultraperformance liquid chromatography determination of anthraquinones in dietary supplements. J Agric Food Chem 62:8822–8829
Yan YY, Chen X, Hu S, Tian J, Bai XH (2014) Simultaneous preconcentration and analysis of anthraquinones based on ultrasound emulsification ionic liquid microextraction. J Chromatogr Sci 52:218–225
Zhang LS, Xing RR, Hu S, Chen X, Bai XH (2014) Novel multiple-solvent simultaneous microextraction for flavonoid and anthraquinone preconcentration in traditional Chinese medicine. Anal Methods 6:1076–1081
Zhang LS, Hu S, Chen X, Bai XH, Li QS (2013) A new ionic liquid-water-organic solvent three phase microextraction for simultaneous preconcentration flavonoids and anthraquinones from traditional Chinese prescription. J Pharm Biomed Anal 86:36–39
Shi Z, Jiang H, Hu J, Li Z, Zhang H (2014) Dispersive liquid-liquid microextraction and high-performance liquid chromatographic determination of anthraquinone derivatives in human urine after oral administration of San-Huang tablets. J Liq Chromatogr Relat Technol 37:2062–2071
Zhong HF, Shi YP (2010) Temperature-assisted ionic liquid dispersive liquid-liquid microextraction combined with high performance liquid chromatography for the determination of anthraquinones in Radix et Rhizoma Rhei samples. Talanta 82:1010–1016
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 81973287) and Natural Science Foundation of Shanxi Province (Grant No. 201701D121145).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animal performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hu, S., Zhang, Sm., Wang, Cl. et al. Reverse Micelle Hollow Fiber Liquid-Phase Microextraction Coupled with HPLC for the Determination of Q-Markers of Anthraquinones in Rhubarb and Their Plasma Protein Binding Rates. Chromatographia 83, 757–766 (2020). https://doi.org/10.1007/s10337-020-03888-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-020-03888-x