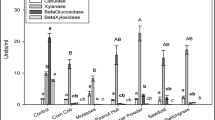
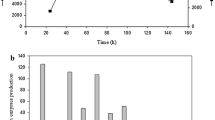
Abstract
β-Glucosidase from novel bacteria Zobellella denitrificans VIT SB117 was isolated, and to increase the production of the enzyme, various growth parameters of the bacteria were optimized. Plackett–Burman design and response surface methodology helped determine the most significant parameters (fructose, temperature, and culture volume) resulting in a 10-fold increase in enzyme activity. The enzyme was purified and kinetics study for free and immobilized enzyme revealed Km of 4.76 mM and 8.39 mM, Kcat of 255.02 s−1 and 114.02 s−1, and Vmax of 4.33 mg/s and 1.25 mg/s, respectively. Enzyme characterization determined optimum substrate concentration and incubation time as 3.5 mM and 10 min respectively for the free enzyme, and 4 mM and 20 min respectively for the immobilized enzyme for maximum activity. pH 5, 45 °C incubation temperature and addition of Mg2+ and Mn2+ ions exhibited similar stimulatory effects on free and immobilized enzyme activities while Hg2+ ions showed strong inhibitory effects. The immobilized enzyme had negligible loss of activity after a month’s storage at 2–4 °C in acetate buffer and ~ 27.76% residual activity after 17 continuous cycles. These optimized parameters were employed for bioethanol production from lignocellulosic wastes. A total cellulose recovery of 52.77% was achieved after pretreatment. Release of ~ 57 mg/g substrate reducing sugars was achieved by enzymatic hydrolysis using immobilized cellulase enzyme complex that produced ~ 5.46 mg/ml bioethanol after 144 h of fermentation using yeast.







Similar content being viewed by others
References
Saravanan AP, Mathimani T, Deviram G, Rajendran K, Pugazhendhi A (2018) Biofuel policy in India: a review of policy barriers in sustainable marketing of biofuel. J Clean Prod 193:734–747. https://doi.org/10.1016/j.jclepro.2018.05.033
Toor M, Kumar SS, Malyan SK, Bishnoi NR, Mathimani T, Rajendran K, Pugazhendhi A (2020) An overview on bioethanol production from lignocellulosic feedstocks. Chemosphere 242:125080. https://doi.org/10.1016/j.chemosphere.2019.125080
Arun N, Dalai AK (2020) Environmental and socioeconomic impact assessment of biofuels from lignocellulosic biomass. In: Lignocellulosic Biomass to Liquid Biofuels. Academic Press, pp. 283–299. https://doi.org/10.1016/B978-0-12-815936-1.00009-5
Azhar SHM, Abdulla R, Jambo SA, Marbawi H, Gansau JA, Faik AAM, Rodrigues KF (2017) Yeasts in sustainable bioethanol production: a review. Biochem Biophys Rep 10:52–61. https://doi.org/10.1016/j.bbrep.2017.03.003
Derman E, Abdulla R, Marbawi H, Sabullah MK (2018) Oil palm empty fruit bunches as a promising feedstock for bioethanol production in Malaysia. Renew Energy 129:285–298. https://doi.org/10.1016/j.renene.2018.06.003
Jambo SA, Abdulla R, Marbawi H, Gansau JA (2019) Response surface optimization of bioethanol production from third generation feedstock-Eucheuma cottonii. Renew Energy 132:1–10. https://doi.org/10.1016/j.renene.2018.07.133
Dharmaraja J, Shobana S, Arvindnarayan S, Vadivel M, Atabani AE, Pugazhendhi A, Kumar G (2020) Biobutanol from lignocellulosic biomass: bioprocess strategies. In Lignocellulosic biomass to liquid biofuels. Academic Press, pp. 169-193. https://doi.org/10.1016/B978-0-12-815936-1.00005-8
Carrillo-Nieves D, Alanís MJR, de la Cruz QR, Ruiz HA, Iqbal HM, Parra-Saldivar R (2019) Current status and future trends of bioethanol production from agro-industrial wastes in Mexico. Renew Sust Energ Rev 102:63–74. https://doi.org/10.1016/j.rser.2018.11.031
Bhardwaj V, Degrassi G, Bhardwaj RK (2017) Bioconversion of cellulosic materials by the action of microbial cellulases. Int Res J Eng Technol 4(8):494–503
Srivastava N, Rathour R, Jha S, Pandey K, Srivastava M, Thakur VK, Sengar RS, Gupta VK, Mazumder PB, Khan AF, Mishra PK (2019) Microbial beta glucosidase enzymes: recent advances in biomass conversation for biofuels application. Biomolecules 9(6):220. https://doi.org/10.3390/biom9060220
Li D, Li X, Dang W, Tran PL, Park SH, Oh BC, Hong W, Lee J, Park KH (2013) Characterization and application of an acidophilic and thermostable β-glucosidase from Thermofilum pendens. J Biosci Bioeng 115(5):490–496. https://doi.org/10.1016/j.jbiosc.2012.11.009
Singhania RR, Patel AK, Sukumaran RK, Larroche C, Pandey A (2013) Role and significance of beta-glucosidases in the hydrolysis of cellulose for bioethanol production. Bioresour Technol 127:500–507. https://doi.org/10.1016/j.biortech.2012.09.012
Ahmed A, Nasim FUH, Batool K, Bibi A (2017) Microbial β-glucosidase: sources, production and applications. J Appl Environ Microbiol 5(1):31–46
Mahapatra S, Vickram AS, Sridharan TB, Parameswari R, Pathy MR (2016) Screening, production, optimization and characterization of β-glucosidase using microbes from shellfish waste. 3. Biotech 6(2):213. https://doi.org/10.1007/s13205-016-0530-7
Sørensen A, Lübeck M, Lübeck P, Ahring B (2013) Fungal beta-glucosidases: a bottleneck in industrial use of lignocellulosic materials. Biomolecules 3(3):612–631. https://doi.org/10.3390/biom3030612
Chang J, Park IH, Lee YS, Ahn SC, Zhou Y, Choi YL (2011) Cloning, expression, and characterization of β-glucosidase from Exiguobacterium sp. DAU5 and transglycosylation activity. Biotechnol Bioprocess Eng 16(1):97–106. https://doi.org/10.1007/s12257-010-0092-1
Joshi SM, Waghmare JS, Sonawane KD, Waghmare SR (2015) Bio-ethanol and bio-butanol production from orange peel waste. Biofuels 6(1–2):55–61. https://doi.org/10.1080/17597269.2015.1045276
Mohit SM, Chandrashekhar B, Tanushree C, Kanwal S (2011) Production of bio-ethanol from Jatropha oilseed cakes via dilute acid hydrolysis and fermentation by Saccharomyces cerevisiae. Int J Biotechnoly Appl 3(1):441–447
Chandrashekhar B, Mishra MS, Sharma K, Dubey S (2011) Bio-ethanol production from textile cotton waste via dilute acid hydrolysis and fermentation by Saccharomyces cerevisiae. J Ecobiotechnol 3(4):06–09
Wu YW, Shao Y, Khanipov K, Golovko G, Pimenova M, Fofanov Y, Chu KH (2017) Draft genome sequence of Zobellella denitrificans ZD1 (JCM 13380), a salt-tolerant denitrifying bacterium capable of producing poly (3-hydroxybutyrate). Genome Announcements 5(36):e00948–e00917. https://doi.org/10.1128/genomeA.00948-17
Liew KJ, Teo SC, Shamsir MS, Sani RK, Chong CS, Chan KG, Goh KM (2018) Complete genome sequence of Rhodothermaceae bacterium RA with cellulolytic and xylanolytic activities. 3. Biotech 8(8):376–378. https://doi.org/10.1007/s13205-018-1391-z
Gouripur G, Kaliwal B (2017) Screening and optimization of β-glucosidase producing newly isolated Lactobacillus plantarum strain LSP-24 from colostrum milk. Biocatal Agric Biotechnol 11:89–96. https://doi.org/10.1016/j.bcab.2017.06.007
Kumar S, Haq I, Prakash J, Raj A (2017) Improved enzyme properties upon glutaraldehyde cross-linking of alginate entrapped xylanase from Bacillus licheniformis. Int J Biol Macromol 98:24–33. https://doi.org/10.1016/j.ijbiomac.2017.01.104
Gupta A, Kumar V, Dubey A, Verma AK (2014) Kinetic characterization and effect of immobilized thermostable β-glucosidase in alginate gel beads on sugarcane juice. ISRN Biochem 2014. https://doi.org/10.1155/2014/178498
Ratanapongleka K, Punbut S (2018) Removal of acetaminophen in water by laccase immobilized in barium alginate. Environ Technol 39(3):336–345. https://doi.org/10.1080/09593330.2017.1301563
Amin F, Bhatti HN, Bilal M, Asgher M (2017) Improvement of activity, thermo-stability and fruit juice clarification characteristics of fungal exo-polygalacturonase. Int J Biol Macromol 95:974–984. https://doi.org/10.1016/j.ijbiomac.2016.10.086
de Cassia PJ, Giese EC, de Souza Moretti MM, dos Santos Gomes AC, Perrone OM, Boscolo M, da Silva R, Gomes E, Martins DAB (2017) Effect of metal ions, chemical agents and organic compounds on lignocellulolytic enzymes activities. Enzyme Inhibitors Activators:139–164 https://doi.org/10.5772/65934
Olajuyigbe FM, Nlekerem CM, Ogunyewo OA (2016) Production and characterization of highly thermostable β-glucosidase during the biodegradation of methyl cellulose by Fusarium oxysporum. Biochem Res Int 2016. https://doi.org/10.1155/2016/3978124
Liew KJ, Lim L, Woo HY, Chan KG, Shamsir MS, Goh KM (2018) Purification and characterization of a novel GH1 beta-glucosidase from Jeotgalibacillus malaysiensis. Int J Biol Macromol 115:1094–1102. https://doi.org/10.1016/j.ijbiomac.2018.04.156
Chen K, Jing X, Liao H (2018) Kinetic models and effects of Mn (II) ion on ethanol production from cornstalks. BioResources 13(1):954–966
Bilal M, Zhao Y, Noreen S, Shah SZH, Bharagava RN, Iqbal HM (2019) Modifying bio-catalytic properties of enzymes for efficient biocatalysis: a review from immobilization strategies viewpoint. Biocatal Biotransform 37(3):159–182. https://doi.org/10.1080/10242422.2018.1564744
Mohammadi M, Heshmati MK, Sarabandi K, Fathi M, Lim LT, Hamishehkar H (2019) Activated alginate-montmorillonite beads as an efficient carrier for pectinase immobilization. Int J Biol Macromol 137:253–260. https://doi.org/10.1016/j.ijbiomac.2019.06.236
Jameel AT, Maalim KY, Yusof F (2019) Relative characterization of immobilized beta-glucosidase on calcium-alginate and acid functionalized multiwalled carbon nanotubes. J Teknol 81(3). https://doi.org/10.11113/jt.v81.13314
Singh G, Verma AK, Kumar V (2016) Catalytic properties, functional attributes and industrial applications of β-glucosidases. 3. Biotech 6(1):3. https://doi.org/10.1007/s13205-015-0328-z
Patil RA, Deshannavar UB (2017) Dry sugarcane leaves: renewable biomass resources for making briquettes. Int J Eng Res Technol 10(1):232–235
Srivastava AK, Agrawal P, Rahiman A (2014) Delignification of rice husk and production of bioethanol. Int J Innov Res Sci Eng Technol 3(3):10187–10194
Rech FR, Fontana RC, Rosa CA, Camassola M, Ayub MAZ, Dillon AJ (2019) Fermentation of hexoses and pentoses from sugarcane bagasse hydrolysates into ethanol by Spathaspora hagerdaliae. Bioprocess Biosyst Eng 42(1):83–92. https://doi.org/10.1007/s00449-018-2016-y
Funding
We would like to thank the management of Vellore Institute of Technology, Vellore, India, for providing us with necessary seed funds to carry out our research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mahapatra, S., Manian, R. Enhancement, production, and immobilization of beta-glucosidase from Zobellella denitrificans VIT SB117 and its utilization in bioethanol production from lignocellulosic feedstock. Biomass Conv. Bioref. 12, 447–458 (2022). https://doi.org/10.1007/s13399-020-00718-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00718-w