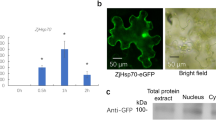
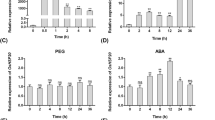
Abstract
Organisms can produce heat shock proteins (HSPs) in response to elevated temperatures and other abiotic stresses. However, the function of HSPs, including small heat shock proteins (sHSPs), in stress tolerance is not fully explored. To improve our understanding of sHSPs, we isolated a gene of sHSPs from David lily (Lilium davidii Duchartre) called LimHSP16.45. Results reveal that LimHSP16.45 is a cytosolic class II sHSP. The HSP17.6II of Arabidopsis thaliana belongs to the HSP20 chaperone protein and is similar to Lim16.45HSP of David lily in terms of size and structure. Both genes have a small heat shock-like alpha-crystallin domain (ACD) structure. To further study the function of Lim16.45HSP, we overexpress it in Arabidopsis hsp17.6II mutant. Then, we detect the expression of LimHSP16.45 in transgenic plant under abiotic stresses and analyze the heat tolerance of transgenic plant. In addition, we measure the activity of three antioxidant enzymes (peroxidase, catalase and superoxide dismutase) and the content of soluble sugar and proline in transgenic plants under abiotic stresses.We found that transgenic plant is tolerant to heat and oxidative stresses given its increased survival rate relative to the hsp17.6II and wild type. Moreover, the content of soluble sugar and proline considerably increase in the transgenic plant. These results support the positive role of LimHSP16.45 in response to heat stress in plants. We suspect that LimHSP16.45 enhances plant tolerance to abiotic stresses by stimulating the activity of ROS-scavenging enzymes and other protective enzymes and increasing the synthesis of proline.







Similar content being viewed by others
REFERENCES
Zhu, J.K., Salt and drought stress signal transduction in plants, Annu. Rev. Plant Biol., 2002, vol. 53, p. 247.
Kotak, S., Larkindale, J., Lee, U., von Koskull-Doring, P., Vierling, E., and Scharf, K.D., Complexity of the heat stress response in plants, Curr. Opin. Plant B-iol., 2007, vol. 10, p. 310.
Lindquist, S., The heat-shock response, Annu. Rev. Biochem., 2007, vol. 55, p. 1151.
Cheng, G., Basha, E., Wysocki, V.H., and Vierling, E., Insights into small heat shock protein and substrate structure during chaperone action derived from hydrogen/deuterium exchange and mass spectrometry, J. B-iol. Chem., 2008, vol. 283, p. 26634.
Stengel, F., Baldwin, A.J., Painter, A.J., Jaya, N., Basha, E., Kay, L.E., Vierling, E., Robinson, C.V., and Benesch, J.L., Quaternary dynamics and plasticity underlie small heat shock protein chaperone function, Proc. Natl. Acad. Sci. USA, 2010, vol. 107, p. 2007.
Basha, E., Jones, C., Blackwell, A.E., Cheng, G., Waters, E.R., Samsel, K.A., Siddique, M., Pett, V., Wysocki, V., and Vierling, E., An unusual dimeric small heat shock protein provides insight into the mechanism of this class of chaperones, J. Mol. Biol., 2013, vol. 425, p. 1683.
Charng, Y.Y., Liu, H.C., Liu, N.Y., Hsu, F.C., and Ko, S.S., Arabidopsis Hsa32, a novel heat shock protein, is essential for acquired thermotolerance during long recovery after acclimation, Plant Physiol., 2006, vol. 14, p. 1297.
Zwirowski, S., Klosowska, A., Obuchowski, I., Nillegoda, N.B., Piróg, A., Zieztkiewicz, S., Bukau, B., Mogk, A., and Liberek, K., Hsp70 displaces small heat shock proteins from aggregates to initiate protein refolding, EMBO J., 2017, vol. 36, p. 783.
Rampino, P., Mita, G., Assab, E., de Pascali, M., Giangrande, E., Treglia, A.S., and Perrotta, C., Two sunflower 17.6HSP genes, arranged in tandem and highly homologous, are induced differently by various elicitors, Plant Biol., 2009, vol. 12, p. 13.
Sun, W., Bernard, C., van de Cotte, B., van Montagu, M., and Verbruggen, N., At-HSP17.6A, encoding a small heat-shock protein in Arabidopsis, can enhance osmotolerance upon overexpression, Plant J., 2001, vol. 27, p. 407.
Volkov, R.A., Panchuk, I.I., Mullineaux, P.M., and Schoffl, F., Heat stress-induced H2O2 is required for effective expression of heat shock genes in Arabidopsis,Plant Mol. Biol., 2006, vol. 61, p. 733.
Sanmiya, K., Suzuki, K., Egawa, Y., and Shono, M., Mitochondrial small heat-shock protein enhances thermotolerance in tobacco plants, FEBS Lett., 2004, vol. 557, p. 265.
Qiu, X.B., Shao, Y.M., Miao, S., and Wang, L., The diversity of the DnaJ/Hs40 family, the crucial partners for Hsp70 chaperones, Cell. Mol. Life Sci., 2006, vol. 63, p. 2560.
Haslbeck, M. and Vierling, E., A first line of stress defense: small heat shock proteins and their function in protein homeostasis, J. Mol. Biol., 2015, vol. 427, p. 1537.
Nakamoto, H. and Vígh, L., The small heat shock proteins and their clients, Cell. Mol. Life Sci., 2007, vol. 64, p. 294.
Eyles, S.J. and Gierasch, L.M., Nature’s molecular sponges: small heat shock proteins grow into their chaperone roles, Proc. Natl. Acad. Sci. USA, 2010, vol. 107, p. 2727.
Richter, K., Haslbeck, M., and Buchner, J., The heat shock response: life on the verge of death, Mol. Cell, 2010, vol. 40, p. 253.
Friedrich, K.L., Giese, K.C., Buan, N.R., and Vierling, E., Interactions between small heat shock protein subunits and substrate in small heat shock protein–substrate complexes, J. Biol. Chem., 2004, vol. 279, p. 1080.
Basha, E., Lee, G.J., Breci, L.A., Hausrath, A.C., Buan, N.R., Giese, K.C., and Vierling, E., The identity of proteins associated with a small heat shock protein during heat stress in vivo indicates that these chaperones protect a wide range of cellular functions, J. Biol. Chem., 2004, vol. 279, p. 7566.
Giese, K.C., Basha, E., Catague, B.Y., and Vierling, E., Evidence for an essential function of the N terminus of a small heat shock protein in vivo, independent of in vitro chaperone activity, Proc. Natl. Acad. Sci. USA, 2005, vol. 102, p. 18896.
Basha, E., Friedrich, K.L., and Vierling, E., The N‑terminal arm of small heat shock proteins is important for both chaperone activity and substrate specificity, J. Biol. Chem., 2006, vol. 281, p. 39943.
Balogi, Z., Török, Z., Balogh, G., Jósvay, K., Shigapova, N., Vierling, E., Vígh, L., and Horváth, I., “Heat shock lipid” in cyanobacteria during heat/light-acclimation, Arch. Biochem. Biophys., 2005, vol. 436, p. 346.
Morrow, G. and Tanguay, R.M., Drosophila melanogaster Hs22: a mitochondrial small heat shock protein influencing the aging process, Front. Genet., 2015, vol. 6: 1026.
Fleckenstein, T., Kastenmuller, A., Stein, M.L., Peters, C., Daake, M., and Krause, M., The chaperone activity of the developmental small heat shock protein Sip1 is regulated by pH-dependent conformational changes, Mol. Cell, 2015, vol. 58, p. 1067.
Vos, M.J., Carra, S., Kanon, B., Bosveld, F., Klauke, K., Sibon, O.C., and Kampinga, H.H., Specific protein homeostatic functions of small heat-shock proteins increase lifespan, Aging Cell, 2015, vol. 15, p. 217.
Reddy, A.S., Ali, G.S., Celesnik, H., and Day, I.S., Coping with stresses: roles of calcium- and calcium/calmodulin-regulated gene expression, Plant Cell, 2011, vol. 23, p. 2010.
Mittler, R., Oxidative stress, antioxidants and stress tolerance, Trends Plant Sci., 2002, vol. 7, p. 1360.
Qu, A.L., Ding, Y.F., Jiang, Q., and Zhu, C., Molecular mechanisms of the plant heat stress response, Biochem. Biophys. Res. Commun., 2013, vol. 432, p. 203.
Rizhsky, L., Davletova, S., Liang, H., and Mittler, R., The zinc finger protein Zat12 is required for cytosolic ascorbate peroxidase 1 expression during oxidative stress in Arabidopsis,J. Biol. Chem., 2004, vol. 279, p. 11736.
Mu, C., Zhang, S., Yu, G., Chen, N., Li, X., and Liu, H., Overexpression of small heat shock protein LimHSP16.45 in Arabidopsis enhances tolerance to abiotic stresses, PLoS One, 2013, vol. 8: e82264. https://doi.org/10.1371/journal.pone.0082264
ACKNOWLEDGMENTS
Thanks to Ministry of Education Key Laboratory of Cell Activities and Stress Adaptations, School of Life Sciences, Lanzhou University.
Funding
This work was financially supported by grants from the National Natural Science Foundation of China (project nos. 31770326 and 31300229), and Fundamental Research Funds for the Central Universities (project no. lzujbky-2017-149).
Author information
Authors and Affiliations
Contributions
Changjun Mu, Ruizhen Yang, and Xiaofeng Li designed the experiments; Ruizhen Yang and Guanzhong Yu performed most of the experiments; Haojie Li interpreted data and generated figures; Changjun Mu and Ruizhen Yang wrote the manuscript.
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants as objects of research.
Additional information
Abbreviations: ACD—alpha-crystallin domain; APX—ascorbate peroxidase; CAT—catalase; HSGs—heat shock granules; HSP—heat shock protein; POD—peroxidase; ROSs—reactive oxygen species; sHSP—small heat shock protein; SOD—superoxide dismutase; WT—wild type.
Supplementary material
Rights and permissions
About this article
Cite this article
Yang, R., Yu, G., Li, H. et al. Overexpression of Small Heat Shock Protein LimHSP16.45 in Arabidopsis hsp17.6II Mutant Enhances Tolerance to Abiotic Stresses. Russ J Plant Physiol 67, 231–241 (2020). https://doi.org/10.1134/S102144372002017X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S102144372002017X