Abstract
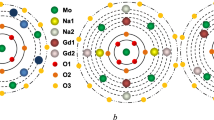
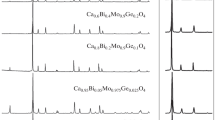
A concentration series of sodium–gadolinium molybdate single crystals have been grown by the Czochralski method from melts of stoichiometric and some nonstoichiometric compositions in atmospheres with different oxygen contents. The actual compositions, microhardness, and crack resistance of the grown crystals have been investigated. All crystals are characterized by a significant sodium deficit with respect to stoichiometry and a large number of vacancies in the (Na + Gd) sublattice. The range of congruent melting is determined for this compound, and its homogeneity range is estimated to extend (on the scale of atomic concentration ratios Gd/Na) at least from 1.10 to 1.75. It is found that the microhardness of sodium– gadolinium molybdate crystals is significantly anisotropic, whereas the degree of crack resistance anisotropy does not exceed the measurement error. At the same time, neither microhardness nor crack resistance exhibit any significant dependence on the growth charge composition and the synthesis conditions in the investigated range of variation in these parameters.



Similar content being viewed by others
REFERENCES
A. Garcia-Cortes, C. Cascales, A. de Andres, et al., IEEE J. Quantum Electron. 43 (2), 157 (2007).
S. N. Ushakov, V. A. Romanyuk, P. A. Ryabochkina, et al., Kvantovaya Elektron. 40 (6), 475 (2010).
A. García-Cortés, J. M. Cano-Torres, X. Han, et al., J. Appl. Phys. 101 (6), 063110 (2007).
A. Schmidt, S. Rivier, V. Petrov, et al., J. Opt. Soc. Am. B: Opt. Phys. 25 (8), 1341 (2008).
A. A. Kaminskii, H. J. Eichler, K. Ueda, et al., Appl. Opt. 38 (21), 4533 (1999).
E. V. Zharikov, D. A. Lis, A. V. Popov, et al., Kvantovaya Elektron. 36 (6), 515 (2006).
A. Garcia-Cortes, J. M. Cano-Torres, M. D. Serrano, et al., IEEE J. Quantum Electron. 43, 758 (2007).
A. A. Lagatsky, X. Han, M. D. Serrano, et al., Opt. Lett. 35, 3027 (2010).
E. V. Zharikov, D. A. Lis, A. M. Onishchenko, et al., Kvantovaya Elektron. 36 (1), 39 (2006).
A. Arakcheeva, D. Logvinovich, G. Chapuis, et al., Chem. Sci. 3, 384 (2012).
C. Zhao, X. Yin, F. Huang, et al., J. Solid State Chem. 184, 3190 (2011).
C. Cascales, A. Mendez-Blas, M. Rico, et al., Opt. Mater. 27, 1672 (2005).
V. K. Trunov, V. A. Efremov, and Yu. A. Velikodnyi, Crystal Chemistry and Properties of Double Molybdates and Tungstates (Nauka, Leningrad, 1986) [in Russian], p. 171.
P. A. Ryabochkina, S. A. Antoshkina, S. A. Klimin, et al., J. Lumin. 138, 32 (2013).
F. A. Bolschikov, G. M. Kuz’micheva, D. A. Lis, et al., J. Cryst. Growth 311 (17), 4171 (2009).
L. D. Merkle, M. Dubinskii, B. Zandi, et al., Opt. Mater. 27, 343 (2004).
E. V. Zharikov, D. A. Lis, K. A. Subbotin, et al., Acta Phys. Pol. A 124 (2), 274 (2013).
V. Volkov, M. Rico, A. Mendez-Blas, et al., J. Phys. Chem. Solids 63, 95 (2002).
J. Fan, H. Zhang, J. Wang, et al., J. Phys. D: Appl. Phys. 39, 1041 (2006).
A. Mendez-Blas, V. Volkov, C. Cascales, et al., J. Alloys Compd. 323–324, 315 (2001).
E. Ya. Rode, V. N. Karpov, and M. M. Ivanova, Zh. Neorg. Khim. 16, 1713 (1971).
T. M. Rybakova, Extended Abstract of Cand. Sci. Dissertation in Chemistry (Moscow State University, Moscow, 1974), p. 16.
A. A. Maier, M. V. Provotorov, and V. A. Balashov, Usp. Khim. 42, 1788 (1973).
E. Ya. Rode, G. A. Balagina, M. M. Ivanova, et al., Zh. Neorg. Khim. 13, 762 (1968).
W. W. Ge, H. J. Zhang, J. Y. Wang, et al., J. Appl. Phys. 98, 013542 (2005).
E. E. Dunaeva, L. I. Ivleva, G. M. Kuz’micheva, et al., Proc. LVII Int. Conf. “Urgent Problems of Strength,” Sevastopol, May 24–27,2016, p. 124.
G. M. Kuz’micheva, E. A. Zagorul’ko, N. B. Bolotina, et al., Crystallogr. Rep. 59 (1), 22 (2014).
K. A. Subbotin, D. A. Lis, V. V. Slavkina, et al., The 18-th Int. Conf. on Crystal Growth and Epitaxy “ICCGE-18,” Nagoya, Japan, August 7–12,2016, Poster presentation TuP-G06-24.
X. Lu, Z. You, J. Li, et al., Solid State Commun. 146, 292 (2008).
W. Zhao, Z. Lin, L. Zhang, et al., J. Alloys Compd. 509 (6), 2815 (2011).
V. Morozov, A. Arakcheeva, B. Redkin, et al., Inorg. Chem. 51 (9), 5324 (2012).
G. M. Kuz’micheva, I. A. Kaurova, V. B. Rybakov, et al., Cryst. Eng. Commun. 18, 2928 (2016).
K. M. Tumanyan, M. M. Gevorkyan, and R. E. Voskanyan, USSR Inventor’s Certificate no. SU 1082875 A (June 16, 1982), p. 3.
K. Niihara, R. Morena, and D. P. H. Hasselman, J. Mater. Sci. Lett. 1, 13 (1982).
V. B. Dudnikova and E. V. Zharikov, Phys. Solid State 59 (5), 866 (2017).
G. M. Kuz’micheva, V. B. Rybakov, K. A. Subbotin, et al., Russ. J. Inorg. Chem. 57 (8), 1128 (2012).
G. B. Lutts, A. L. Denisov, E. V. Zharikov, et al., Opt. Quantum Electron. 22, 269 (1990).
R. D. Shannon, Acta Crystallogr. A 32 (6), 751 (1976).
G. R. Anstis, P. Chantikul, B. R. Lawn, et al., J. Am. Ceram. Soc. 64 (9), 533 (1981).
A. V. Vinogradov, V. A. Lomonov, Yu. A. Pershin, et al., Crystallogr. Rep. 47 (6), 1036 (2002).
M. Yu. Gryaznov, S. V. Shotin, V. N. Chuvil’deev, et al., Crystallogr. Rep. 57 (1), 144 (2012).
Funding
This study was supported by the Russian Science Foundation, project no. 18-12-00517.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by Yu. Sin’kov
Rights and permissions
About this article
Cite this article
Subbotin, K.A., Titov, A.I., Lis, D.A. et al. Growth, Compositions, and Mechanical Characteristics of Sodium–Gadolinium Molybdate Single Crystals. Crystallogr. Rep. 65, 182–190 (2020). https://doi.org/10.1134/S106377452002025X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106377452002025X