Abstract
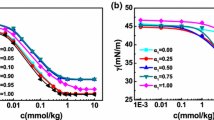
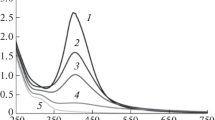
Stable silver nanoparticles have been obtained in reverse micelles of known and available nonionic surfactants, sorbitan monooleate (Span 80) and tetraethoxylated dodecanol and p-nonylphenol (Brij 30 and Tergitol NP-4, respectively), in n-decane. The obtained nanoparticles have been characterized using a number of physicochemical methods. The stability, hydrodynamic diameter, and electrokinetic potential of the nanoparticles have been studied as depending on the amounts of added water; chloroform; and an anionic surfactant, sodium bis(2-ethylhexyl)sulfosuccinate (AOT). It has been shown that, for different surfactants, AOT should be introduced in different ways to provide the nanoparticles with the electrokinetic potential values sufficient for enhancing their stability and ensuring the possibility of concentrating them by nonaqueous electrophoresis. In the case of Brij 30, it is preferable to introduce AOT after the synthesis as a “charging additive,” while, in the case of Tergitol NP-4, it should be added as a cosurfactant at the stage of the synthesis. The nanoparticles synthesized in Span 80 micelles have a rather high electrokinetic potential even in the absence of the additives. In this case, the use of AOT as a cosurfactant decreases the electrokinetic potential of the nanoparticles, while, being added after the synthesis, it increases the potential.



Similar content being viewed by others
REFERENCES
Wu, W., Nanoscale, 2017, vol. 9, p. 7342.
Kamyshny, A. and Magdassi, S., Small, 2014, vol. 10, p. 3515.
Shin, D.-H., Woo, S., Yem, H., Cha, M., Cho, S., Kang, M., Jeong, S., Kim, Y., Kang, K., and Piao, Y., ACS Appl. Mater. Interfaces, 2014, vol. 6, p. 3312.
Vassem, M., McKerricher, G., and Shamim, A., ACS Appl. Mater. Interfaces, 2016, vol. 8, p. 177.
Jason, N.J., Shen, W., and Cheng, W., ACS Appl. Mater. Interfaces, 2015, vol. 7, p. 16760.
Lee, K.J., Jun, B.H., Kim, T.H., and Joung, J., Nanotechnology, 2006, vol. 17, p. 2424.
Shinde, S.R., Banpurkar, A.G., Adhi, K.P., Limaye, A.V., Ogale, S.B., Date, S.K., and Marest, G., Mod. Phys. Lett. B, 1996, vol. 10, p. 1517.
Begin-Colin, S., Wolf, F., and Le Caer, G., J. Phys. III, 1997, vol. 7, p. 473.
Ananthapadmanabhan, P.V., Sreekumar, K.P., Venkatramani, N., Sinha, P.K., and Taylor, P.R., J. Alloys Compd., 1996, vol. 244, p. 70.
Lu, J., Yang, H., Yu, S., and Zou, G., Mater. Chem. Phys., 1996, vol. 45, p. 197.
Pileni, M.P., J. Phys. Chem., 1993, vol. 97, p. 6961.
Eastoe, J., Hollamby, M.J., and Hudson, L., Adv. Colloid Interface Sci., 2006, vols. 128–130, p. 5.
Zhang, W., Qiao, X., Chen, J., and Wang, H., J. Colloid Interface Sci., 2006, vol. 302, p. 370.
Popovetskiy, P.S., Shaparenko, N.O., Arymbaeva, A.T., and Bulavchenko, A.I., Colloid J., 2016, vol. 78, p. 485.
Sainis, S.K., Merrill, J.W., and Duffresne, E.R., Langmuir, 2008, vol. 24, p. 13334.
Sainis, S.K., Germain, V., Mejean, C.O., and Duffresne, E.R., Langmuir, 2008, vol. 24, p. 1160.
Popovetskiy, P.S., Bulavchenko, A.I., and Manakov, A.Yu., Opt. Zh., 2011, vol. 78, no. 7, p. 67.
Bulavchenko, A.I. and Pletnev, D.N., J. Phys. Chem. C, 2008, vol. 112, p. 16365.
Bulavchenko, A.I. and Popovetskiy, P.S., Langmuir, 2010, vol. 26, p. 736.
O’Brien, R.W. and White, L.R., J. Chem. Soc., Faraday Trans., 1978, vol. 74, p. 1607.
Delgado, A.V., Gonzalez-Caballero, F., Hunter, R.J., Koopal, L.K., and Lyklema, J., J. Colloid Interface Sci., 2007, vol. 309, p. 194.
Morrison, I.D., Colloids Surf. A, 2008, vol. 71, p. 1.
Popovetskiy, P.S., Bulavchenko, A.I., Demidova, M.G., and Podlipskaya, T.Yu., Colloid J., 2015, vol. 77, p. 58.
Popovetskiy, P.S. and Beketova, D.I., Colloids Surf. A, 2019, vol. 568, p. 51.
Funding
This work was supported by the Russian Foundation for Basic Research, project no. 18-33-00064 mol-a.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declare that he have no conflict of interest.
Additional information
Translated by A. Kirilin
Rights and permissions
About this article
Cite this article
Popovetskiy, P.S. Synthesis and Characterization of Silver Nanoparticles in Reverse Micelles of Nonionic Surfactants and in Their Mixed Micelles with AOT. Colloid J 82, 144–151 (2020). https://doi.org/10.1134/S1061933X2002009X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X2002009X