Abstract
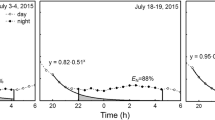
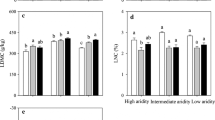
Regulation of leaf gas exchange plays an important role in the survival of trees and shrubs under future climate change. However, the responses of leaf water potential and gas exchange of shrubs in semi-arid areas to the precipitation alteration are not clear. Here, we conducted a manipulated experiment with three levels of precipitation, i.e., a control with ambient precipitation, 50% above ambient precipitation (irrigation treatment), and 50% below ambient precipitation (drought treatment), with two common shrubs, Salix psammophila C. Wang & C. Y. Yang (isohydric plant, maintaining a constant leaf water potential by stomatal regulation) and Caragana korshinskii Kom. (anisohydric plant, having more variable leaf water potential), on the Chinese Loess Plateau in 2014 and 2015. We measured the seasonal variations of predawn and midday leaf water potential (Ωpd and Ωmd), two parameters of gas exchange, i.e., light-saturated assimilation (An) and stomatal conductance (gs), and other foliar and canopy traits. The isohydric S. psammophila had a similar An and a higher gs than the anisohydric C. korshinskii under drought treatment in 2015, inconsistent with the view that photosynthetic capacity of anisohydric plants is higher than isohydric plants under severe drought. The two shrubs differently responded to precipitation manipulation. Ωpd, An and gs were higher under irrigation treatment than control for S. psammophila, and these three variables and Ωmd were significantly higher under irrigation treatment and lower under drought treatment than control for C. korshinskii. Leaf water potential and gas exchange responded to manipulated precipitation more strongly for C. korshinskii than for S. psammophila. However, precipitation manipulation did not alter the sensitivity of leaf gas exchange to vapor-pressure deficit and soil moisture in these two shrubs. Acclimation to long-term changes in soil moisture in these two shrubs was primarily attributed to the changes in leaf or canopy structure rather than leaf gas exchange. These findings will be useful for modeling canopy water-carbon exchange and elucidating the adaptive strategies of these two shrubs to future changes in precipitation.
Similar content being viewed by others
References
Adams H D, Guardiola-Claramonte M, Barron-Gafford G A, et al. 2009. Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought. Proceedings of the National Academy of Sciences of the United States of America, 106(17): 7063–7066.
Ai S H, Li Y Y, Chen L R. 2017. Responses of the shoot growth in Salix psammophila and Caragana korshinskii to manipulated precipitation variation. Science of Soil and Water Conservation, 15(3): 90–98. (in Chinese)
Chaves M M, Pereira J S, Maroco J, et al. 2002. How plants cope with water stress in the field? Photosynthesis and growth. Annals of Botany, 89(7): 907–916.
de Dios V R, Fischer C, Colinas C. 2007. Climate change effects on Mediterranean forests and preventive measures. New Forest, 33(1): 29–40.
Dong X J, Zhang X S. 2001. Some observations of the adaptations of sandy shrubs to the arid environment in the Mu Us Sandland: leaf water relations and anatomic features. Journal of Arid Environments, 48(1): 41–48. i]Falster D S, Warton D I, Wright I J. 2006. tUsers Guide to SMATR: Standardised Major Axis Tests & Routines Version 2.0. [2014-03-11]. http://www.bio.mq.edu.au/ecology/SMATR/.
Fan L M. 2007. Groundwater seepage caused by mining and the prevention strategies in the northern Shaanxi. Mining Safety and Environmental Protection, 34(5): 62–64. (in Chinese)
Flexas J, Bota J, Loreto F, et al. 2004. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biology, 6(3): 269–279.
Garcia-Forner N, Adams H D, Sevanto S, et al. 2016. Responses of two semiarid conifer tree species to reduced precipitation and warming reveal new perspectives for stomatal regulation. Plant, Cell and Environment, 39(1): 38–49.
Gulías J, Flexas J, Mus M, et al. 2003. Relationship between maximum leaf photosynthesis, nitrogen content and specific leaf area in Balearic endemic and non-endemic Mediterranean species. Annals of Botany, 92(2): 215–222.
Hartmann H, Ziegler W, Kolle O, et al. 2013. Thirst beats hunger-declining hydration during drought prevents carbon starvation in Norway spruce saplings. New Phytologist, 200(2): 340–349.
Huxman T E, Smith M D, Fay P A, et al. 2004. Convergence across biomes to a common rain-use efficiency. Nature, 429(6992): 651–654.
Jiang G M, Zhu G J. 2001. Effects of natural high temperature and irradiation on photosynthesis and related parameters in three arid sandy shrub species. Acta Phytoecologia Sinica, 25(5): 525–531. (in Chinese)
Lamont B B, Groom P K, Cowling R M. 2002. High leaf mass per area of related species assemblages may reflect low rainfall and carbon isotope discrimination rather than low phosphorus and nitrogen concentrations. Functional Ecology, 16(3): 403–412.
Li Y Y, Chen W Y, Chen J C, et al. 2016. Vulnerability to drought-induced cavitation in shoots of two typical shrubs in the southern Mu Us Sandy Land, China. Journal of Arid Land, 8(1): 125–137.
Limousin J M, Misson L, Lavoir A V, et al. 2010. Do photosynthetic limitations of evergreen Quercus ilex leaves change with long-term increased drought severity? Plant, Cell and Environment, 33(5): 863–875.
Limousin J M, Bickford C P, Dickman L T, et al. 2013. Regulation and acclimation of leaf gas exchange in a piñon-juniper woodland exposed to three different precipitation regimes. Plant, Cell and Environment, 36(10): 1812–1825.
Liu H Y, Li J Y, Zhao Y, et al. 2007. Influence of drought stress on gas exchange and water use efficiency of Salix psammophila growing in five places. Arid Zone Research, 24(6): 815–820. (in Chinese)
Liu J, He X, Bao H L, et al. 2010. Distribution of fine roots of Salix psammophila and its relationship with soil moisture in Mu Us Sandland. Journal of Desert Research, 30(6): 1362–1366. (in Chinese)
Lovisolo C, Perrone I, Carra A, et al. 2010. Drought-induced changes in development and function of grapevine (Vitis spp.) organs and in their hydraulic and non-hydraulic interactions at the whole-plant level: a physiological and molecular update. Functional Plant Biology, 37(2): 98–116.
Ma C C, Gao Y B, Guo H Y, et al. 2008. Physiological adaptations of four dominant Caragana species in the desert region of the Inner Mongolia Plateau. Journal of Arid Environments, 72(3): 247–254.
Martínez-Vilalta J, Garcia-Forner N. 2016. Water potential regulation, stomatal behaviour and hydraulic transport under drought: deconstructing the iso/anisohydric concept. Plant, Cell and Environment, 40(6): 962–976.
Martin-StPaul N K, Limousin J M, Rodriguez-Calcerrada J, et al. 2012. Photosynthetic sensitivity to drought varies among populations of Quercus ilex along a rainfall gradient. Functional Plant Biology, 39(1): 25–37.
Maseda P H, Fernández R J. 2006. Stay wet or else: three ways in which plants can adjust hydraulically to their environment. Journal of Experimental Botany, 57(15): 3963–3977.
McDowell N G, Pockman W T, Allen C D, et al. 2008. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytologist, 178(4): 719–739.
Misson L, Limousin J M, Rodriguez R, et al. 2010. Leaf physiological responses to extreme droughts in Mediterranean Quercus ilex forest. Plant, Cell and Environment, 33(11): 1898–1910.
Murray F W. 1967. On the computation of saturation vapor pressure. Journal of Applied Meteorology, 58(6): 203–204.
Niu X W, Ding Y C, Zhang Q, et al. 2003. Studies on the characteristics of Caragana root development and some relevant physiology. Acta Botania Boreal-Occidential Sinica, 23(5): 860–865. (in Chinese)
Nogués S, Alegre L. 2002. An increase in water deficit has no impact on the photosynthetic capacity of field-grown Mediterranean plants. Functional Plant Biology, 29(5): 621–630.
Oren R, Sperry J S, Katul G G, et al. 1999. Survey and synthesis of intra- and interspecific variation in stomatal sensitivity to vapor pressure deficit. Plant, Cell and Environment, 22(12): 1515–1526.
Quero J L, Sterck FJ, Martínez-Vilalta J, et al. 2011. Water-use strategies of six co-existing Mediterranean woody species during a summer drought. Oecologia, 166: 45–57.
Ripullone F, Guerrieri M R, Nole A, et al. 2007. Stomatal conductance and leaf water potential responses to hydraulic conductance variation in Pinus pinaster seedlings. Trees, 21(3): 371–378.
Ripullone F, Borghetti M, Raddi S, et al. 2009. Physiological and structural changes in response to altered precipitation regimes in a Mediterranean macchia ecosystem. Trees, 23(4): 823–834.
Sevanto S, Mcdowell N G, Dickman L T, et al. 2014. How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant, Cell and Environment, 37(1): 153–161.
Tang K L, Hou Q C, Wang B K, et al. 1993. The environment background and administration way of wind-water erosion crisscross region and Shenmu experimental area on the Loess Plateau. Memoir of Northwestern Institute of Soil and Water Conservation, Academia Sinica and Ministry of Water Conservancy, 18: 1–15. (in Chinese)
Tardieu F, Simonneau T. 1998. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modeling isohydric and anisohydric behaviours. Journal of Experimental Botany, 49(Special Issue): 419–432.
Weltzin J F, Loik M E, Schwinning S, et al. 2003. Assessing the response of terrestrial ecosystems to potential changes in precipitation. Bioscience, 53(10): 941–952.
Xu D H, Fang X W, Bin Z J, et al. 2012. Eco-physiological mechanism of Caragana korshinskii Kom adaptation to extreme drought stress: leaf abscission and keeping chloroplast integrity in stem. Journal of Desert Research, 32(3): 691–697. (in Chinese)
Zhou S X, Medlyn B E, Prentice I C. 2016. Long-term water stress leads to acclimation of drought sensitivity of photosynthetic capacity in xeric but not riparian Eucalyptus species. Annals of Botany, 117(1): 133–144.
Zhu Y J, Jia Z Q, Lu Q, et al. 2010. Water use strategy of five shrubs in Ulanbuh Desert. Scientia Silvae Sinicae, 46(4): 15–21. (in Chinese)
Acknowledgements
The study was funded by the National Natural Science Foundation of China (41571130082, 41371507).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Chen, J., Ai, S. et al. Responses of leaf water potential and gas exchange to the precipitation manipulation in two shrubs on the Chinese Loess Plateau. J. Arid Land 12, 267–282 (2020). https://doi.org/10.1007/s40333-020-0008-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40333-020-0008-7