Abstract
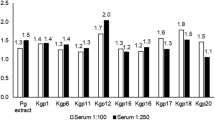
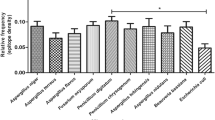
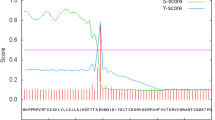
Gout is a common rheumatic condition caused due to increase in serum uric acid level (hyperuricemia). Uricase is for lowering the level of uric acid but unfortunately, it is not produced in humans due to evolutionary changes. Therefore, it is administered to humans from outside in case of the high uric acid level in blood. A different formulation of uricase from bacterial, fungal, and mammalian sources is present in the market for the treatment of hyperuricemia conditions. Uricase formulation showed immunogenic response due to the occurrence of hypersensitivity reaction during the treatment that results in poor patient compliance. The purpose of this study was to clarify the variation of Uricase immunogenicity from different sources. We have used some immunoinformatic approaches to analyze and understand some structural aspects of immunogenic and allergenic epitopes of Uricase by calculation of relative frequency for eleven global alleles. As per our knowledge, this is the first immunoinformatic study of Uricase (structural based immunogenicity prediction) that deciphered the high immunogenic nature of Uricase but no significant difference in immunogenicity was found among Uricase isolated from Aspergillus flavus, Bacillus subtillis, and mammalian source. This study gives a further lead to develop some methods (include bioengineering of less immunogenic version of the uricase or utilizing the homologous enzymes) for minimizing immune response or search new sources of uricase that could be less or non-immunogenic.








Similar content being viewed by others
Abbreviations
- SUA:
-
Serum uric acid
- FDA:
-
Food and drug administration
- IEDB:
-
Immune epitope database
- MHC:
-
Major histocompatibility complex
References
Yainoy S, Phuadraksa T, Wichit S et al (2019) Production and characterization of recombinant wild type uricase from indonesian coelacanth (L. menadoensis) and improvement of its thermostability by in silico rational design and disulphide bridges engineering. Int J Mol Sci 20:1–20. https://doi.org/10.3390/ijms20061269
Schlesinger N (2008) Hyperuricemia, gout, and diet. Nutr Rheum Dis. https://doi.org/10.1007/978-1-59745-403-2_10
S Ebrahimi, M Shafiei, A Ghasemian, SKS Mostafavi, RJ Nojoomi Cloning expression assessment of uricase gene from. 10:672–677
Borghi C (2017) The management of hyperuricemia: back to the pathophysiology of uric acid. Curr Med Res Opin 33:1–4. https://doi.org/10.1080/03007995.2017.1378502
De Oliveira EP, Burini RC (2012) High plasma uric acid concentration: Causes and consequences. Diabetol Metab Syndr 4:1–7. https://doi.org/10.1186/1758-5996-4-12
Imani M, Shahmohamadnejad S (2017) Recombinant production of Aspergillus flavus uricase and investigation of its thermal stability in the presence of raffinose and lactose. 3 Biotech 7:1–9. https://doi.org/10.1007/s13205-017-0841-3
Lotfy WA (2008) Production of a thermostable uricase by a novel Bacillus thermocatenulatus strain. Bioresour Technol 99:699–702. https://doi.org/10.1016/j.biortech.2007.01.048
Pawar SV, Rathod VK (2018) Optimization of novel and greener approach for the coproduction of uricase and alkaline protease in Bacillus licheniformis by Box-Behnken model. Prep Biochem Biotechnol 48:24–33. https://doi.org/10.1080/10826068.2017.1381623
Ueng S (2005) Rasburicase (Elitek): a novel agent for tumor lysis Syndrome. Baylor Univ Med Cent Proc 18:275–279. https://doi.org/10.1080/08998280.2005.11928082
Parente V, Corti S (2017) When myasthenia gravis is deemed refractory: clinical signposts and treatment strategies. Ther Adv Neurol Disord 23:153–156. https://doi.org/10.1177/https
Kotb E (2016) Improvement of uricase production from Bacillus subtilis RNZ-79 by solid state fermentation of shrimp shell wastes. Biol 71:229–238. https://doi.org/10.1515/biolog-2016-0040
Pfrimer P, De Moraes LMP, Galdino AS et al (2010) Cloning, purification, and partial characterization of bacillus subtilis urate oxidase expressed in escherichia coli. J Biomed Biotechnol. https://doi.org/10.1155/2010/674908
Wipfler-Freißmuth E, Dejaco C, Duftner C et al (2009) Urate oxidase (rasburicase) for treatment of severe acute gout: a case report. Clin Exp Rheumatol 27:658–660
Shannon JA, Cole SW (2012) Pegloticasa: un agente novel para el tratamiento de gota refractaria. Ann Pharmacother 46:368–376. https://doi.org/10.1345/aph.1Q593
Garay RP, El-Gewely MR, Labaune JP, Richette P (2012) Therapeutic perspectives on uricases for gout. Jt Bone Spine 79:237–242. https://doi.org/10.1016/j.jbspin.2012.01.004
Nyborg AC, Ward C, Zacco A et al (2016) A therapeutic uricase with reduced immunogenicity risk and improved development properties. PLoS ONE 11:1–23. https://doi.org/10.1371/journal.pone.0167935
Andreatta M, Nielsen M (2018) Bioinformatics tools for the prediction of T-cell epitopes. Methods Mol Biol 1785:269–281. https://doi.org/10.1007/978-1-4939-7841-0_18
Panda SK, Mahapatra RK (2017) In-silico screening, identification and validation of a novel vaccine candidate in the fight against Plasmodium falciparum. Parasitol Res 116:1293–1305. https://doi.org/10.1007/s00436-017-5408-z
Dhanda SK, Karosiene E, Edwards L et al (2018) Predicting HLA CD4 immunogenicity in human populations. Front Immunol 9:1–14. https://doi.org/10.3389/fimmu.2018.01369
Retailleau P, Colloc’h N, Vivarès D et al (2004) Complexed and ligand-free high-resolution structures of urate oxidase (Uox) from Aspergillus flavus: a reassignment of the active-site binding mode. Acta Crystallogr Sect D 60:453–462. https://doi.org/10.1107/S0907444903029718
Jiang YY Structure of urate oxidase from Bacillus subtilis 168.
Kratzer JT, Lanaspa MA, Murphy MN et al (2014) Evolutionary history and metabolic insights of ancient mammalian uricases. Proc Natl Acad Sci USA 111:3763–3768. https://doi.org/10.1073/pnas.1320393111
Jespersen MC, Peters B, Nielsen M, Marcatili P (2017) BepiPred-2.0: Improving sequence-based B-cell epitope prediction using conformational epitopes. Nucleic Acids Res 45:W24–W29. https://doi.org/10.1093/nar/gkx346
Ponomarenko J, Bui HH, Li W et al (2008) ElliPro: a new structure-based tool for the prediction of antibody epitopes. BMC Bioinform 9:1–8. https://doi.org/10.1186/1471-2105-9-514
Wang P, Sidney J, Dow C et al (2008) A systematic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. PLoS Comput Biol. https://doi.org/10.1371/journal.pcbi.1000048
Wang P, Sidney J, Kim Y et al (2010) Peptide binding predictions for HLA, DR, DP and DQ molecules. BMC Bioinform 11:568. https://doi.org/10.1186/1471-2105-11-568
Andreatta M, Karosiene E, Rasmussen M et al (2015) Accurate pan-specific prediction of peptide-MHC class II binding affinity with improved binding core identification. Immunogenetics 67:641–650. https://doi.org/10.1007/s00251-015-0873-y
Nielsen M, Lundegaard C, Lund O (2007) Prediction of MHC class II binding affinity using SMM-align, a novel stabilization matrix alignment method. BMC Bioinform 8:1–12. https://doi.org/10.1186/1471-2105-8-238
Nielsen M, Lund O (2009) NN-align. An artificial neural network-based alignment algorithm for MHC class II peptide binding prediction. BMC Bioinform 10:296. https://doi.org/10.1186/1471-2105-10-296
Sidney J, Assarsson E, Moore C et al (2008) Quantitative peptide binding motifs for 19 human and mouse MHC class i molecules derived using positional scanning combinatorial peptide libraries. Immunome Res 4:1–14. https://doi.org/10.1186/1745-7580-4-2
Sturniolo T, Bono E, Ding J et al (1999) Generation of tissue-specific and promiscuous HLA ligand databases using DNA microarrays and virtual HLA class II matrices. Nat Biotechnol 17:555–561. https://doi.org/10.1038/9858
Gonzalez-Galarza FF, Christmas S, Middleton D, Jones AR (2011) Allele frequency net: a database and online repository for immune gene frequencies in worldwide populations. Nucleic Acids Res 39:913–919. https://doi.org/10.1093/nar/gkq1128
Schulze zur Wiesch J, Lauer GM, Day CL et al (2005) Broad repertoire of the CD4 + Th cell response in spontaneously controlled hepatitis C virus infection includes dominant and highly promiscuous epitopes. J Immunol 175:3603–3613. https://doi.org/10.4049/jimmunol.175.6.3603
Moutaftsi M, Peters B, Pasquetto V et al (2006) A consensus epitope prediction approach identifies the breadth of murine TCD8+-cell responses to vaccinia virus. Nat Biotechnol 24:817–819. https://doi.org/10.1038/nbt1215
Dimitrov I, Bangov I, Flower DR, Doytchinova I (2014) AllerTOP vol 2—a server for in silico prediction of allergens. J Mol Model. https://doi.org/10.1007/s00894-014-2278-5
Feldmann M, Howard JG, Desaymard C (1975) Role of Antigen structure in the discrimination between tolerance and immunity by B cells. Immunol Rev 23:78–97. https://doi.org/10.1111/j.1600-065X.1975.tb00150.x
Bachmann MF, Rohrer UH, Kündig TM et al (1993) The influence of antigen organization on B cell responsiveness. Science 262:1448–1451. https://doi.org/10.1126/science.8248784
Aichele P, Zinke J, Grode L et al (2003) Macrophages of the splenic marginal zone are essential for trapping of blood-borne particulate antigen but dispensable for induction of specific T Cell responses. J Immunol 171:1148–1155. https://doi.org/10.4049/jimmunol.171.3.1148
Liu W, Peng Z, Liu Z et al (2004) High epitope density in a single recombinant protein molecule of the extracellular domain of influenza A virus M2 protein significantly enhances protective immunity. Vaccine 23:366–371. https://doi.org/10.1016/j.vaccine.2004.05.028
Dahiru T (2011) P-Value, a true test of statistical significance a cautionary note. Ann Ibadan Postgrad Med 6:21–26. https://doi.org/10.4314/aipm.v6i1.64038
Of H, Information P, Changes RM et al (2012) Full prescribing information: contents * 1 indications and usage 2 dosage and administration 7. 2 potential for altered drug absorption 8 use in specific populations 3 dosage forms and strengths 4 contraindications 5 warnings and precautions 5. 1 Serio. 1–22
Rasburicase (Elitek). Rasburicase (Elitek) 1–12.
Onda M (2009) Reducing the immunogenicity of protein therapeutics. Curr Drug Targets 10:131–139. https://doi.org/10.2174/138945009787354511
Anderson A, Singh JA (2010) Pegloticase for chronic gout (review) summary of findings for the main comparison. Cochrane DB Syst Rev. https://doi.org/10.1002/14651858.CD008335.pub2
Masclee G (2014) Anti-inflammatory and antipyretic analgesics and drugs used in gout. Elsevier, Amsterdam
Guttmann A, Krasnokutsky S, Pillinger MH, Berhanu A (2017) Pegloticase in gout treatment-safety issues, latest evidence and clinical considerations. Ther Adv Drug Saf. https://doi.org/10.1177/2042098617727714
Acknowledgements
Author thanks National Institute of Technology, Raipur, India for continuous support and assistance during research work & scientific writing. The authors have no financing to disclose.
Funding
There are no funders to report for this submission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have declared that they don’t have a conflict of interest in publishing the research.
Research Involving Human Participants and/or Animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tripathi, S., Parmar, J. & Kumar, A. Structure-Based Immunogenicity Prediction of Uricase from Fungal (Aspergillus flavus), Bacterial (Bacillus subtillis) and Mammalian Sources Using Immunoinformatic Approach. Protein J 39, 133–144 (2020). https://doi.org/10.1007/s10930-020-09886-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-020-09886-0